Many attempts have been made over the decades to develop predictive models for the presence of obstructive sleep apnoea (OSA) within the community and for gauging the severity of the condition in order to more accurately assess responses to a variety of treatments, ranging from continuous positive airway pressure to weight loss strategies. None of these models have succeeded in demonstrating the necessary sensitivities and specificities to be deployed in real practice although they may occasionally be helpful in highlighting the possible existence of obstructive sleep apnoea (OSA) in non-sleep medicine clinics, e.g. surgical clinics, cardiology clinics.1
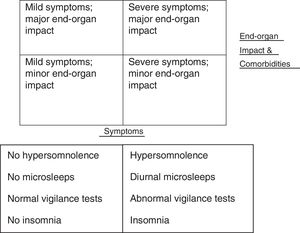
Obstructive sleep apnoea hypopnoea syndrome (OSAHS) is a complex disease which can present in a variety of different ways and contribute to the development of a number of comorbidities (see Fig. 1). This complexity may explain why even some of the most creative attempts at predicting and simplifying diagnosis have been failed.2
On a clinical level, history and examination alone, including blood pressure and body mass index (BMI) measurement can predict the presence of OSAHS in about 50% of patients attending a specialised sleep clinic.3 However, definitive diagnosis requires objective monitoring during sleep using either polygraphy or polysomnography. When screening for OSAHS either in clinical settings or the general population, several algorithms have been developed over the years to more accurately identify its presence in patients presenting with suggestive complaints. These include using a combination of clinical variables such as BMI, neck circumference, jaw structure, the presence of snoring, reports of nocturnal breathing disturbances and the presence of hypertension. Whilst the sensitivity of these approaches may be high (78-95%) the specificity remains low (41-63%).4 A recent meta-analysis of screening questionnaires used in a total of 1,484 subjects 5included 4 studies utilising the Berlin questionnaire,6 2 studies using the Wisconsin Sleep Questionnaire,7 1 study using the STOP questionnaire 8 and one the STOP-BANG questionnaire.5 Four studies of patients referred to a sleep clinic arrived at a pooled sensitivity of 73% (95% CI, 66-78%) for the diagnosis of OSAHS and a pooled specificity of 61% (95% CI, 55-67%). Six studies on patients without symptoms of OSA revealed a pooled sensitivity of 77% (95% CI, 73-80%) for detecting OSAHS and a pooled specificity of 53% (95% CI, 50-57%).5 Questionnaires devised over the last 25 years to evaluate both sleepiness and quality of life in OSAHS have included the Functional Outcomes of Sleep Questionnaire,10 the Calgary Sleep Apnoea Quality of Life Index (SAQLI),11 the short SAQLI 12and the Epworth Sleepiness Score.13 The latter is probably the most widely utilised questionnaire world-wide in the practice of sleep medicine. Unfortunately, correlation with objective measures of sleepiness is poor and the questions themselves very open to misinterpretation.12 Even the pESS 14developed to obviate some of these problems carries the same degree of concerns regarding the accuracy with which respondents note their answers.
In Pataka's et al.15 paper published in this issue, an attempt was made to combine a number of well-established questionnaires used for screening OSAHS with the number and degree of oxygen dips using oximetry, verified using attended polysomnography. Neither the Berlin Questionnaire,6 nor the STOP-BANG questionnaire 9were found to be useful which is unsurprising in a population of both males and females with suspected OSAHS. The sensitivity and specificity of oximetry was found to be high, but again, this too is hardly surprising in the context of a supervised in-lab study. A recent publication, addressing the existing publications on sleep questionnaires and sleep diaries,16 discussed the possible cost reduction of using questionnaires when compared to Type I – Type III. This may explain the current widespread interest in sleep questionnaires. At present there appears to be a tendency in primary care medicine to use questionnaires and not a standardized history in assessing for the presence or absence of OSAHS. While this method is a time- and therefore cost-saving procedure, it remains to be proven if such an approach will really reduce public spending due to its low specificity.
Oximetry is used in many centres as a screening tool for the presence of OSA in combination with a clinical history or suspicion for the disorder, there are certain caveats that need to be borne in mind. Not all oximeters are created equal, despite great advances in technology in recent times. There is also no minimal specification on signal processing in the context of sleep breathing disorders either nationally or internationally. The minimum standard criteria set by the AASM are a sampling rate of 25Hz with averaging of 3 values.17 A resolution of 0.1% is desirable but not specified and device specific signalling processing can differ substantially among oximeters.18 An understanding of SpO2 waveforms morphologies is essential in order to interpret the output appropriately, as is a knowledge of what is normal and abnormal throughout the lifespan.19 Automated systems may not be reliable and there may be problems with blood flow, haemoglobinopathies and tissue optics in the very obese. Movement artefact can also affect the readings depending on whether the study is attended or unattended and measurement inaccuracies of±2% SpO2 should be factored into the outputs. A large number of studies have been undertaken over the decades in OSA patients suggesting a 31-100% sensitivity and 41-100% specificity;20 information which suggests just how unreliable the technique can be. Finally, a normal SpO2 does not exclude OSA as many hypopnoeas and apnoeas may not be accompanied by desaturation and an abnormal SpO2 does not automatically signify OSA.
Pataka et al. deployed in their study continuous positive airways pressure (CPAP) therapy in their moderate-severe OSAHS patients (AHI≥15) or in those with an AHI≥5 with symptoms of daytime sleepiness, irrespective of the manner in which they were identified. This is in keeping with current treatment strategies although CPAP does not uniformly improve symptoms in unselected patients with OSAHS, nor consistently prevent or improve cardiometabolic abnormalities.2 Pataka's et al. study confirms that CPAP therapy itself is important as part of the diagnostic pathway in establishing the relationship between symptom profile and OSA irrespective of how the diagnosis was arrived at. Should symptoms not improve despite adequate CPAP compliance, they may be due to other factors which have not been taken into consideration at the outset or which may prove stronger predictors of other therapeutic strategies. We look forward to future studies from this group examining these models.