Biliobronchial fistula (BBF) is a rare condition characterized by communication between the bile duct and the bronchial Tree.1 The first case was described by Peacok's in 1850 in a 20-year-old woman with hepatic echinococcosis.2 Diagnosis is mainly clinical, with radiological or endoscopic support,3 and therapeutic options range from conservative to invasive with highly variable results.4 We present the case of a patient admitted to our service with this unusual disorder.
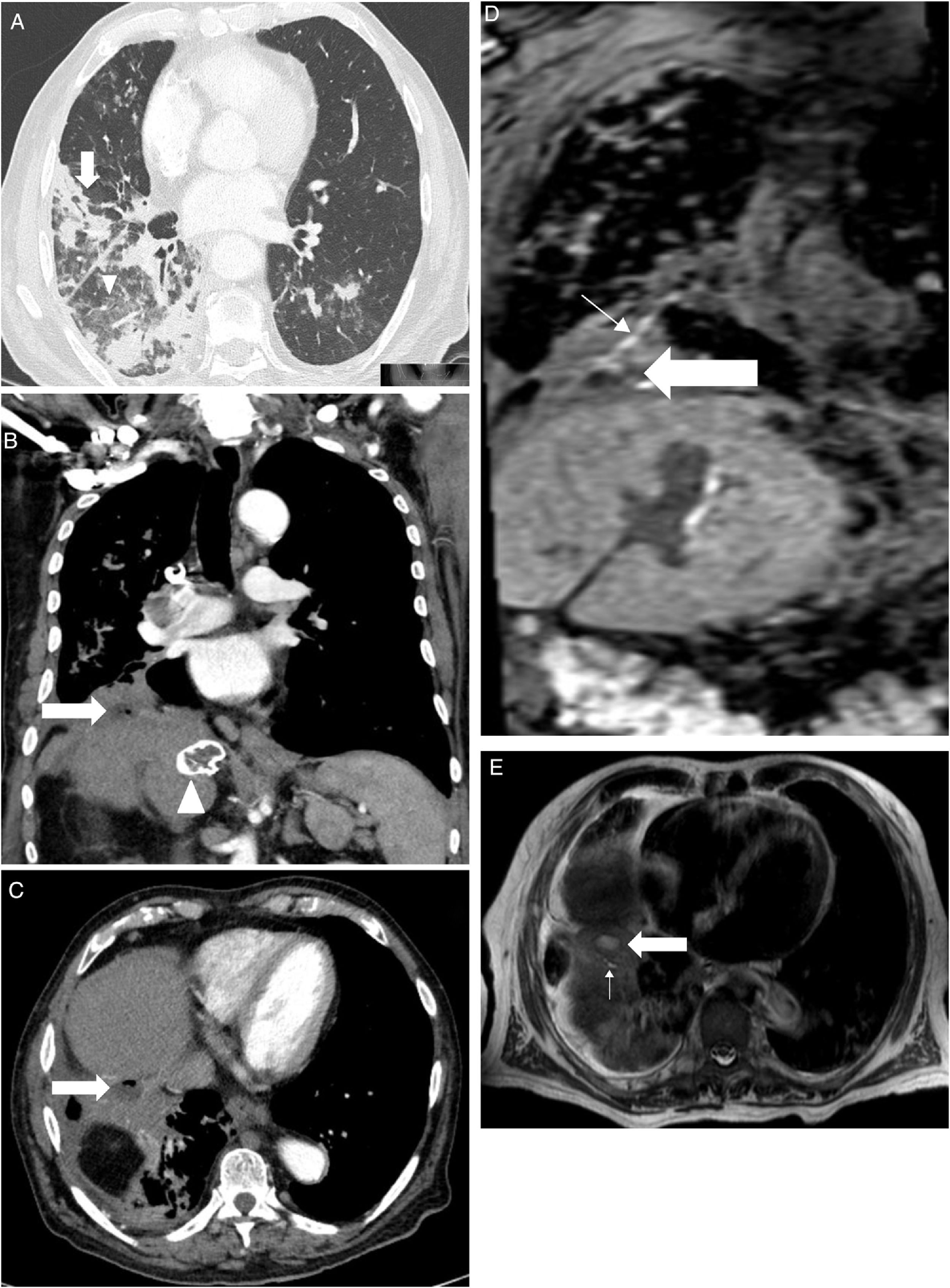
An 88-year-old male was hospitalized with fever, shortness of breath and increased expectoration in the previous month. Fourteen years earlier he had had a partial hepatectomy for a biliary fistula secondary to the removal of a hydatid cyst, with elevation of the right hemidiaphragm as a result of the surgery, and residual lesions to the ipsilateral lung base. He was diagnosed with bronchiectasis with chronic bronchial infection by Pseudomonas aeruginosa and treated with inhaled colistine. In the days prior arriving to the Emergency Department, he had been treated with tobramycin and intravenous amikacin (home hospitalization). On physical examination, he was tachypneic, afebrile, had bilious expectoration, and crackles and hoarseness could be heard throughout the right hemithorax. Blood tests showed leukocytosis (15.110/mm3) with 84% of segmented and 7% of stems; creatinine 1.18mg/dL; Na 129 mmol/L; GGT 83 UI/L; FAL 303 UI/L; total proteins 5.7g/dL; C-reactive protein 24.2mg/dL and procalcitonin 1.32 ng/mL; PaO2 56mm Hg. Bronchofibroscopy showed, from the larynx and throughout the right bronchial tree, abundant yellowish bilious-looking fluid, compatible with bilioptisis, with an inflammatory mucosa of the lower right bronchus lobe without endobronchial lesions. In CT we observed pulmonary infiltrates patched with airborne bronchogram, areas of tarnished glass predominating in the right lower lobe (Figure 1A), a peridiaphragmatic cystic lesion at the base of the right lung with a small air bubble and post-operative liver changes with peripheral calcification in the caudate lobe suggesting a healing hydatid cyst (Figures 1B and 1C). Liver ultrasound showed right hepatectomy with hypertrophy of the left hepatic lobe without biliary lithiasis. Liver MRI and cholangio-MRI scans showed a fistulous tract and subdiaphragmatic abscesses (Figures 1D and 1E). A plastic biliary stent was placed by endoscopic retrograde cholangiography. We also performed a sphincterotomy for biliary decompression. At the same time, the subdiaphragmatic collections in which Pseudomona aeruginosa was cultivated were drained. During the course of antibiotic treatment (ceftazidime and tobramycin), Sthaphylococcus haemolyticus bacteremia occurred, forcing the addition of vancomycin. The patient worsened progressively with renal function deterioration, dying a few days later.
A: Axial cut computerized tomography. Pulmonary infiltrates patched with airborne bronchogram (thick arrow) and areas of tarnished glass predominantly right lower lobe (arrowhead).
Figure 1B: Coronal cut computerized tomography. A small abscess is shown in the diaphragmatic region (thick arrow) and calcified hydatid cyst (arrowhead).
Figure 1C: Axial cut computerized tomography. Abscess (thick arrow) between the posterior hepatic rim and the lower right lobe, with a small air bubble inside.
Figure 1D: Coronal cut of hepatic magnetic resonance imaging in hepato-specific phase with bile duct contrast excretion at the same level as Figure 1B. Abscess (thick arrow) and fistulous path (thin arrow) are shown.
Figure 1E: Axial cut of hepatic magnetic resonance imaging in hepato-specific phase with bile duct contrast excretion at the same level as Figure 1C. Fistulous tract (thin arrow) and abscess (thick arrow) leading to the anterobasal segment of the lower right lobe.
Biliobronchial fistula is a rare condition for which diagnosis is established from the presence of bilioptisis and is confirmed by techniques such as bronchofibroscopy or various imaging studies. It may be congenital or secondary to trauma (most common, including bile duct surgery), liver disease (hydatid cyst and amoebic liver abscess), or bile duct obstruction. A frequent complication in chronic stages, as in our case, is the presence of bronchiectasis in the pulmonary segment involved.5 Occasionally, there may be recurrent pneumonia and ipsilateral pleural effusion, with secondary sepsis, probably caused by chemical pneumonitis that produces bile in the bronchial mucosa. Imaging studies used to confirm the diagnosis (CT scan, ultrasound, hepatic nuclear magnetic resonance, nuclear magnetic resonance, percutaneous cholangiography, and endoscopic retrograde cholangiopancreatography) must demonstrate the fistulous tract.
There is no general agreement on how to deal with BBFs. Ong et al used subcutaneous octreotide and succeeded in reducing bilious cough in a patient and speeding up the closure of the fistula, but its use is limited and is not effective if there is underlying infection, neoplasia or obstruction.6 The definitive treatment is surgical fistulectomy with soft tissue reconstruction, but due to its significant morbidity and mortality and the frequent re-operations it leads to, more conservative interventions have also been proposed aimed at reducing pressure in the biliary tract, such as endoscopic retrograde biliary drainage or percutaneous transhepatic biliary drainage. Depending on the size and location of the BBF, another conservative option would be to try to close the fistula through bronchofibroscopy. The sealing material used should achieve an inflammatory reaction of the mucous membrane that causes the permanent closure of the fistula. There is a wide range of synthetic substances and biological derivatives that can be applied through the flexible bronchoscope.7
What can be learned from this case is that faced with a patient with a history of a traumatic process in the hepatobiliary tract (even if it is old), bilious sputum and bronchiectasis in the right lung, this condition should be suspected.
Author's ContributionsRA, MET, AMdeA and LV were responsible for the conception and design of the study, and wrote and edited the manuscript. All authors read and approved the final manuscript.
FundingThis study was undertaken without funding
Conflicts Of InterestWe wish to confirm that there are no known conflicts of interest associated with this publication. This study did not receive any financial support for this work that could have influenced its outcome. We confirm that the manuscript has been read and approved by all named authors and there are no other persons who satisfied the criteria for authorship who are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us. We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. We confirm that we have followed the regulations of our institutions concerning intellectual property.
We understand that the Corresponding Author is the sole contact for the Editorial process (including Editorial manager and direct communications with the office). She is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs. We confirm that we have provided a current, correct email address which is accessible by the Corresponding Author and which has been configured to accept e-mail from: romina.abelleira.paris@sergas.es
We confirm that the manuscript is not published elsewhere, in any language, and is not under simultaneous consideration by any other journal. Signed by all authors as follows:
Romina Abelleira, María E. Toubes, Anxo Martínez de Alegría and Luis Valdés.