Legionnaire's disease is a pneumonia caused by the gram-negative bacteria Legionella spp. which contaminate artificial freshwater systems. Europe currently faces a rising trend in this pathology which has an incidence of 1 per 100.000 population.1 Although primarily a lung disease, the central nervous system (CNS) may also be affected. The low number of CNS involvement however, might lead physicians to overlook this pathogen agent. The aim of the following report on a Legionnaire's-disease-associated acute meningoencephalitis is to inform clinicians about the heterogeneous presentation of this disease, and consequently improve patient outcome.
Case presentationA 45-year-old male patient who before the time of admission lived independently, was referred to our neurology emergency department with a transient left-sided hemiparesis, an upward gaze deviation and a bilateral vertical and horizontal nystagmus, severe dysarthria, limb and truncal ataxia. In addition, fever with a peak temperature of 41°C was recorded. There were no pathological lung sounds. He had suffered from recurrent episodes of nausea and vomiting for the three days prior to admission. The patient, a breeder of chipmunks and squirrels, was a smoker and had no history of cerebrovascular or epileptic disorders.
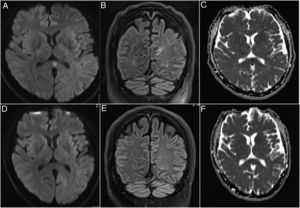
InvestigationsA magnetic resonance imaging (MRI) of the brain upon admission displayed a hyperintensity in the splenium of the corpus callosum and which was completely reversible on day 9 (Fig. 1). Chest CT was consistent with right-lung pneumonia.
Brain MRI imaging of the patient on admission and after 9 days. A brain MRI upon admission showing a hyperintensity in the splenium of the corpus callosum in the diffusion weighted (DWI) (A) and fluid-attenuated inversion recovery (FLAIR) (B) imaging. The apparent diffusion coefficient (ADC) map confirmed the existence of this lesion (C). The pathology was completely reversible on day 9 after admission in the DWI (D), FLAIR (E) and ADC (F) imaging.
Laboratory tests on the day of admission demonstrated a blood leucocyte count of 10.9×109/L, a C-reactive protein of 359.9mg/dL, and an Interleukin-2 receptor of 800U/mL. Creatinine was 1.38mg/dL; creatine kinase 453U/L; aspartate aminotransferase 74U/L; alanine transaminase 53U/L; gamma-glutamyl transferase 108U/L; and blood sodium 126mmol/L. Cerebrospinal fluid (CSF) showed a pleocytosis of 9 cells/μL, while protein, lactate, and glucose concentrations were normal. The CSF cytology was compatible with a necrotic process. Blood and CSF cultures, virological and tuberculosis testing, as well as toxicological and autoimmune encephalitis antibody examinations remained negative. Eventually, an antigen test in the urine and a bronchial aspirate-PCR were both positive for Legionella pneumophila.
Treatment and outcomeBecause of his severe symptoms, suggesting also a life-threatening involvement of the brainstem, the patient was admitted to our intensive care unit. We immediately initiated an empiric therapy with acyclovir, ampicillin, and ceftriaxone. With no improvement of the clinical situation, the combination of antibiotics was replaced by meropenem. After the isolation of L. pneumophila, levofloxacin was added. Due to the high level of infection parameters, we maintained the therapy regime with meropenem, as we could not rule out a concomitant infection by an additional pathogen. The patient was discharged on day 24 with a remaining ataxia and dysarthria, but has partially regained the ability to eat and drink independently.
DiscussionLegionnaire's disease, a primarily pulmonary pathology, can also manifest neurological symptoms. As the brain is often affected, confusion or an altered mental status are almost ubiquitous.2–5 In addition, brainstem and cerebellar symptoms such as ataxi2,5 or dysarthria,2,5 from which our patient also suffered, are also frequently observed. In a few cases, the disappearance of brainstem reflexes even made mechanical ventilation necessary.5 Hyperintensities are observed at multiple localizations on the brain MRI, although lesions in the splenium do most frequently occur.2,6 The characteristics of these lesions is their full reversibility after initiation of an appropriate antibiotic therapy.2,6 It is hypothesized that this phenomenon is caused by a reversible sinus vein thrombosis.4
CSF remained sterile in most cases.3–6 The striking fact that in most patients Legionella spp. was not detectable in the CSF has led some authors to consider the underlying mechanism to be an immune- or toxin-mediated reaction rather than direct damage by the bacteria.3 Some authors detected the pathogen with more elaborate techniques and it is possible that by applying some of them, the bacteria would also have been isolated.7 We did not pursue these steps for a lack of therapeutic consequences.
Finally, we would like to tie in with the reflections of Charles et al., that due to the paucity of knowledge of this topic,7 and in our case also the missing cultural proof of a Legionella spp. in the CSF, we are not able to be certain about the etiology of the pathological state. Indeed, the observed hyponatremia might have contributed to the patient's neurological deterioration. Nevertheless, the gradual clinical improvement, as well as the disappearance of the brain hyperintensities after adjusting the antibiotics, encourage us to conclude that Legionnaire's disease is the most probable source for the patient's condition and that the water appliances for the animals he raised were the source of contamination.
ConclusionAlthough primarily belonging to the pulmonary field, Legionnaire's disease displays a wide array of more rare symptoms, among them also neurological ones. As these symptoms are uncommon, physicians might easily overlook this pathogen in cases presenting primarily with extra-pulmonary signs and symptoms. As our multidisciplinary report shows, a constellation of a pneumonia, aseptic CSF, splenial lesions, and contact with water systems should prompt physicians to test for Legionella spp.
Financial supportThere was no financial support for this project.
Conflicts of interestThe authors have no conflicts of interest to declare.
We are grateful to Fabian Berger, Jeffrey Palms, Walter Schulz-Schaeffer and to Michael Kettner for their assistance.