In Portugal, lung cancer (LC) is the first cause of cancer-related death and of death and disability combined. This study aims to analyze the overall survival (OS) and relative survival (RS) of patients diagnosed with LC in 2009–2011 by socio-demographic and tumor characteristics, and analyze sex-specific patterns.
MethodsWe estimated 5-year OS using the Kaplan-Meier method and 5-year net survival through the RS framework. Cox regression modeling was used to determine the hazard ratio (HR) of death associated with each independent variable.
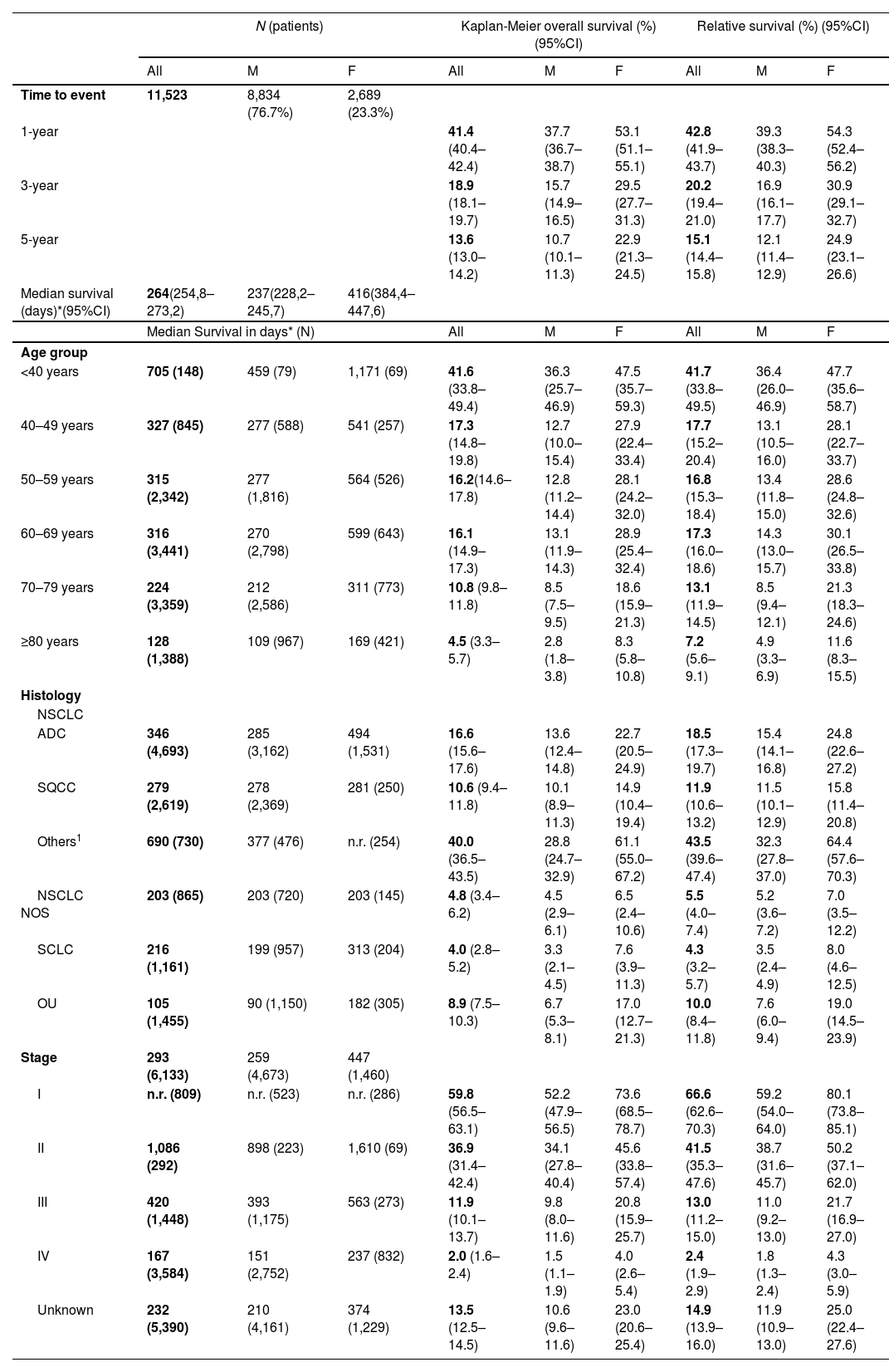
FindingsFor the 11,523 cases analyzed, median 5-year OS was 264 days (95% confidence interval [CI]: 254.8–273.2), the cumulative OS was 13.6% and RS was 15.1%. Males had a lower median survival (237 days; 95% CI: 228.2–245.7) compared to females (416 days; 95% CI: 384.4–447.6) (p < 0.0001) and lower 5-year RS proportions (12.1% vs. 24.9%). RS progressively decreased with age (41.7% for age-group <40 to 7.2% for ≥80) and stage (66.6% for stage I to 2.4% for stage IV). As predictors of decreased survival, we identified male gender, increasing age >50, histologic types (squamous cell carcinoma, non-small cell lung cancer not otherwise specified, other unspecified and small cell lung cancer), and increasing stage. Compared to women, the risk of death in men was 37.7% higher (HR = 1.386; 95% CI: 1.295–1.484).
ConclusionsThe differences between OS and RS were small, reflecting the high lethality of LC. Male gender and older age are factors related to poor prognosis. Histology also plays a role in survival prognosis and varies with gender, but the factor related to the worst survival is stage. Although the study reflects data from a decade ago, and major changes occurred in diagnosis, staging and treatment, particularly for advanced disease, as LC mortality is strongly correlated with late stage diagnosis, all efforts should be made to secure early diagnosis and improve survival prospects.
Lung cancer (LC) is the second most commonly diagnosed cancer (11.4% of the total cases) and the first cause of cancer death (18.0% of the total cancer deaths).1 In 2019, LC was responsible for 45.8 million (18.2%) DALYs of which 98.8% came from years of life lost (YLL) and 1.2% from years lived with disability (YLDs).2 In Portugal, lung cancer was the first cause of cancer-related death and disability combined and the sixth of all causes in 2019.2
Tobacco smoking is the most important risk factor for LC, along with other environmental pollutants.3-5 The population attributable fraction for tobacco smoking and LC in Portugal in 2018 was 73.2% for incidence (82.6% in men and 51.0% in women) and 74.1% for mortality (84.1% in men and 42.7% in women).6 Nevertheless, only approximately 10% of smokers develop LC, and the disease also occurs in the absence of exposure to cigarette smoke.5
Lung cancer does not become clinically apparent until it reaches an advanced stage and more than 75% of cases are diagnosed when the disease is advanced or metastatic.7,8 The high mortality rate associated with this disease is mostly attributable to late stage at diagnosis, when treatment is less effective and survival rates are considerably lower.9,10
Five-year relative survivals (RS) of 13% and 19% were reported in EUROCARE-5 for patients diagnosed with LC in 2000-2007,11,12 and in the United States (U.S.) from 2009 through 2015, respectively.13 Except for smoking cessation, the highest reduction in LC mortality is achieved by an early diagnosis followed by surgical resection.12 Risk factors for LC could be linked to sex characteristics and/or to some physical and behavioral traits distinct for males or females. An imbalance of these etiologic factors could explain why some LC features may differ between sexes.14
Our study aims to characterize the survival of patients diagnosed with LC in Portugal, by sex, age, tumor histology, and stage at diagnosis, as well as analyze sex-specific patterns in survival. Additionally, we aim to compare overall survival (OS) and RS. To the best of our knowledge, this is the largest study in Portugal to target these objectives using national population-based LC data.
Material and methodsData and sourcesInvasive LC cases (ICD-10 code C34)15 by age and region of residence were obtained for the three-year period 2009 through 2011 from four population-based cancer registries covering the whole country of Portugal. Patients were followed until the end of 2016. Informed consent was not required as data received had been previously anonymized.
The OS and RS analysis were conducted for patients' socio-demographic variables: sex (male/female) and age-groups (<40, 40–49, 50–59, 60–69, 70–79 ≥80) and for tumor characterization variables: stage at diagnosis (I, II, III, IV), and histologic types (non-small cell lung cancer [NSCLC]: non-small cell lung cancer not otherwise specified [NSCLC, NOS], adenocarcinoma [ADC], squamous cell carcinoma [SQCC], and Others; Small cell lung cancer [SCLC]; Other and unspecified [OU]).16 Detailed information about patient characteristics and morphology codes can be found elsewhere.17 We also conducted an analysis stratified by sex to identify differences in survival among males and females.
Statistical methodsAfter descriptive analyses, we estimated 5-year OS using the Kaplan-Meier method, compared by log-rank tests. The primary endpoint used was time to death in 5-year follow-up. The duration of OS was calculated from the date of diagnosis until death or the date of last follow-up. We also estimated five-year net survival through the RS framework using the National Cancer Institute's SEER*Stat software version 8.3.8.18 RS is a standard indicator for comparison of cancer survival in population-based studies for which the underlying cause of death is unknown.11 RS is the ratio of the observed (all-cause) survival to the expected survival of a comparable group of cancer-free individuals.19 RS is useful to enable survival comparison between different populations correcting for differences in non-cancer mortality.20 We calculated five-year RS estimates by the actuarial method. Expected survival probabilities were calculated through the Pohar-Perme estimator21based on regional life expectancy tables.22 We used Cox multiple regression modeling with risk ratios measured by Hazard Ratio (HR) and determined the HR of death associated with each independent variable, adjusted for the effect of all other variables in the equation, considering the corresponding 95% confidence intervals [95%CI].23 For Cox model validation, we used the log likelihood statistic together with the evaluation of the parallelism of the log minus log curves.24 The first model (M1) included the following variables: sex, age, histology and stage. A second model (M2) stratified by sex included age, histology and stage at diagnosis as core variables.
The level of statistical significance was 0.05. Except for RS, the analyses were conducted using Statistical Package for the Social Sciences (IBM SPSS Statistics for Windows, version 26.0, Armonk, NY).
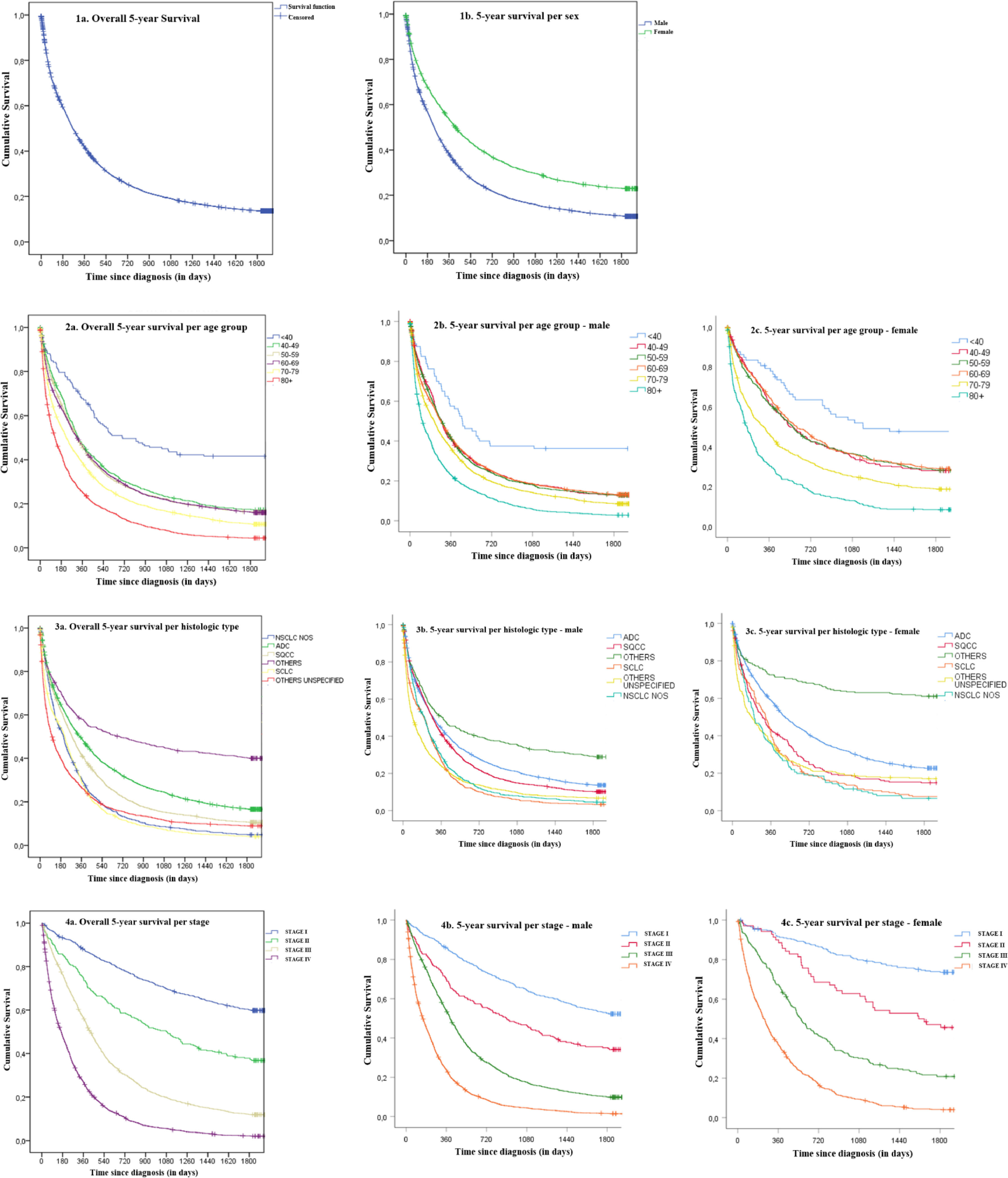
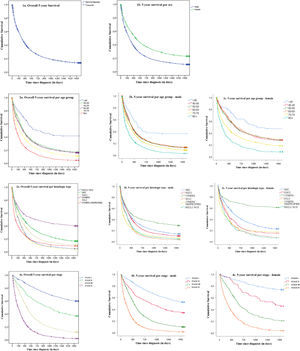
FindingsOverall, there were 11,642 new cases of invasive LC in Portugal in 2009–2011, of which 11,523 cases were included in the survival analysis. Two cases were excluded due to absence of survival information and 117 were lost-to follow-up. There were 76.6% males and 23.4% females, with 91.3% above 50 years-old. NSCLC represented 77.3% of cases. Within NSCLC, ADC was the most frequent histologic type (40.8%), followed by SQCC (22.7%). Locally advanced and metastatic disease accounted for 82% of cases. Median 5-year OS was 264 days (95%CI: 254.8–273.2). Cumulative OS at 1-year, 3-year and 5-year after diagnosis were, respectively, 41.4%, 18.9% and 13.6%. Males presented lower median survival (237 days; 95%CI: 228.2–245.7) compared to females (416 days; 95%CI: 384.4–447.6) (p < 0.0001), as well as lower OS across all time points: 1-year (37.7% vs. 53.1%), 3-year (15.7% vs. 29.5%) and 5-year (10.7% vs. 22.9%) for all stages combined (p < 0.0001). RS at 1-year, 3-year and 5-year were respectively 42.8%, 20.2% and 15.1%. Males presented lower RS compared to females at all times: 1-year (39.3% vs. 54.3%), 3-year (16.9% vs. 30.9%) and 5-year (12.1% vs. 24.9%) (p < 0.0001) (Table 1). Globally the differences between OS and RS were small (ranging from 1.2 to 2.0%).
Overall and relative survival by sex, time since diagnosis, age, histology and stage, Portugal, 2009–2011.
| N (patients) | Kaplan-Meier overall survival (%) (95%CI) | Relative survival (%) (95%CI) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All | M | F | All | M | F | All | M | F | |
| Time to event | 11,523 | 8,834 (76.7%) | 2,689 (23.3%) | ||||||
| 1-year | 41.4 (40.4–42.4) | 37.7 (36.7–38.7) | 53.1 (51.1–55.1) | 42.8 (41.9–43.7) | 39.3 (38.3–40.3) | 54.3 (52.4–56.2) | |||
| 3-year | 18.9 (18.1–19.7) | 15.7 (14.9–16.5) | 29.5 (27.7–31.3) | 20.2 (19.4–21.0) | 16.9 (16.1–17.7) | 30.9 (29.1–32.7) | |||
| 5-year | 13.6 (13.0–14.2) | 10.7 (10.1–11.3) | 22.9 (21.3–24.5) | 15.1 (14.4–15.8) | 12.1 (11.4–12.9) | 24.9 (23.1–26.6) | |||
| Median survival (days)*(95%CI) | 264(254,8–273,2) | 237(228,2–245,7) | 416(384,4–447,6) | ||||||
| Median Survival in days* (N) | All | M | F | All | M | F | |||
| Age group | |||||||||
| <40 years | 705 (148) | 459 (79) | 1,171 (69) | 41.6 (33.8–49.4) | 36.3 (25.7–46.9) | 47.5 (35.7–59.3) | 41.7 (33.8–49.5) | 36.4 (26.0–46.9) | 47.7 (35.6–58.7) |
| 40–49 years | 327 (845) | 277 (588) | 541 (257) | 17.3 (14.8–19.8) | 12.7 (10.0–15.4) | 27.9 (22.4–33.4) | 17.7 (15.2–20.4) | 13.1 (10.5–16.0) | 28.1 (22.7–33.7) |
| 50–59 years | 315 (2,342) | 277 (1,816) | 564 (526) | 16.2(14.6–17.8) | 12.8 (11.2–14.4) | 28.1 (24.2–32.0) | 16.8 (15.3–18.4) | 13.4 (11.8–15.0) | 28.6 (24.8–32.6) |
| 60–69 years | 316 (3,441) | 270 (2,798) | 599 (643) | 16.1 (14.9–17.3) | 13.1 (11.9–14.3) | 28.9 (25.4–32.4) | 17.3 (16.0–18.6) | 14.3 (13.0–15.7) | 30.1 (26.5–33.8) |
| 70–79 years | 224 (3,359) | 212 (2,586) | 311 (773) | 10.8 (9.8–11.8) | 8.5 (7.5–9.5) | 18.6 (15.9–21.3) | 13.1 (11.9–14.5) | 8.5 (9.4–12.1) | 21.3 (18.3–24.6) |
| ≥80 years | 128 (1,388) | 109 (967) | 169 (421) | 4.5 (3.3–5.7) | 2.8 (1.8–3.8) | 8.3 (5.8–10.8) | 7.2 (5.6–9.1) | 4.9 (3.3–6.9) | 11.6 (8.3–15.5) |
| Histology | |||||||||
| NSCLC | |||||||||
| ADC | 346 (4,693) | 285 (3,162) | 494 (1,531) | 16.6 (15.6–17.6) | 13.6 (12.4–14.8) | 22.7 (20.5–24.9) | 18.5 (17.3–19.7) | 15.4 (14.1–16.8) | 24.8 (22.6–27.2) |
| SQCC | 279 (2,619) | 278 (2,369) | 281 (250) | 10.6 (9.4–11.8) | 10.1 (8.9–11.3) | 14.9 (10.4–19.4) | 11.9 (10.6–13.2) | 11.5 (10.1–12.9) | 15.8 (11.4–20.8) |
| Others1 | 690 (730) | 377 (476) | n.r. (254) | 40.0 (36.5–43.5) | 28.8 (24.7–32.9) | 61.1 (55.0–67.2) | 43.5 (39.6–47.4) | 32.3 (27.8–37.0) | 64.4 (57.6–70.3) |
| NSCLC NOS | 203 (865) | 203 (720) | 203 (145) | 4.8 (3.4–6.2) | 4.5 (2.9–6.1) | 6.5 (2.4–10.6) | 5.5 (4.0–7.4) | 5.2 (3.6–7.2) | 7.0 (3.5–12.2) |
| SCLC | 216 (1,161) | 199 (957) | 313 (204) | 4.0 (2.8–5.2) | 3.3 (2.1–4.5) | 7.6 (3.9–11.3) | 4.3 (3.2–5.7) | 3.5 (2.4–4.9) | 8.0 (4.6–12.5) |
| OU | 105 (1,455) | 90 (1,150) | 182 (305) | 8.9 (7.5–10.3) | 6.7 (5.3–8.1) | 17.0 (12.7–21.3) | 10.0 (8.4–11.8) | 7.6 (6.0–9.4) | 19.0 (14.5–23.9) |
| Stage | 293 (6,133) | 259 (4,673) | 447 (1,460) | ||||||
| I | n.r. (809) | n.r. (523) | n.r. (286) | 59.8 (56.5–63.1) | 52.2 (47.9–56.5) | 73.6 (68.5–78.7) | 66.6 (62.6–70.3) | 59.2 (54.0–64.0) | 80.1 (73.8–85.1) |
| II | 1,086 (292) | 898 (223) | 1,610 (69) | 36.9 (31.4–42.4) | 34.1 (27.8–40.4) | 45.6 (33.8–57.4) | 41.5 (35.3–47.6) | 38.7 (31.6–45.7) | 50.2 (37.1–62.0) |
| III | 420 (1,448) | 393 (1,175) | 563 (273) | 11.9 (10.1–13.7) | 9.8 (8.0–11.6) | 20.8 (15.9–25.7) | 13.0 (11.2–15.0) | 11.0 (9.2–13.0) | 21.7 (16.9–27.0) |
| IV | 167 (3,584) | 151 (2,752) | 237 (832) | 2.0 (1.6–2.4) | 1.5 (1.1–1.9) | 4.0 (2.6–5.4) | 2.4 (1.9–2.9) | 1.8 (1.3–2.4) | 4.3 (3.0–5.9) |
| Unknown | 232 (5,390) | 210 (4,161) | 374 (1,229) | 13.5 (12.5–14.5) | 10.6 (9.6–11.6) | 23.0 (20.6–25.4) | 14.9 (13.9–16.0) | 11.9 (10.9–13.0) | 25.0 (22.4–27.6) |
M = male; F = Female; CI = Confidence Interval; ADC = Adenocarcinoma; SQCC = Squamous Cell Carcinoma; NSCLC NOS = Non-Small Cell Lung Cancer Not Otherwise Specified; SCLC = Small Cell Lung Cancer; OU = Other and unspecified; n.r. = not reached
We observed progressive decreases in OS with increasing age, with age-group <40 presenting a survival proportion of 41.6% compared to 4.5% in ≥80. Similar observations occurred for both sexes. The highest decrease was seen between <40 and 40-49 years-old groups with 41.6% vs. 17.3%. RS presented a similar behavior ranging from 41.7% (<40) to 7.2% (≥80).
When addressing histology, a major difference in survival was observed for the NSCLC Otherssubtype with 40.0% 5-year OS, followed by 16.6% in ADC, 10.6% for SQCC and the lowest with 4.0% was SCLC. In men, the highest survival proportion was 28.8% for NSCLC Others, 13.6% for ADC, 10.1% for SQCC and lowest 3.3% for SCLC. For women, the highest survival proportion was again NSCLC Others with 61.1%, 22.7% for ADC, 14.9% for SQCC and the lowest for NSCLC NOS with 6.5%. RS proportions were consistent with OS proportions with differences ranging from 0.3 to 3.5% in total population, 0.2 to 3.5% in males and 0.4 to 3.3% in females. The lowest difference occurred for SCLC and the highest for NSCLC Others across all groups.
The largest differences observed in 5-year OS were related to stage. Specifically, median survival was not reached for stage I (meaning >50% patients alive), regardless of sex, and for stage IV was 167 days for all cases combined, 151 days for males and 237 days for females. Survival proportions markedly decreased with stage, 59.8% for stage I, 36.9% for stage II, 11.9% for stage III and 2% for stage IV. Five-year survival proportions were always higher for women compared to men irrespective of stage: 73.6% vs. 52.2% in stage I, 45.6% vs. 34.1% in stage II, 20.8% vs. 9.8% in stage III and 4.0% vs. 1.5% in stage IV (p < 0.0001). The highest differences between RS and OS were observed for stages I (66.6%; +6.8%) and II (41.5%; +4.6%), but values almost matched for stage IV (2.4%; +0.4%). Similar results obtained for the sex stratification analysis. For cases with unknown stage, we observed a median survival of 232 days (95%CI: 219.3–244.7) that lies between the medians observed for stages III and IV, and a 5-year OS of 13.5%, slightly higher than the observed for stage III. In terms of 5-year RS, we observed 14.9% overall, 11.9% for males and 25.0% for females.
Additional details can be found in Table1. Kaplan-Meier survival curves are presented in Fig. 1.
A multivariable Cox regression model identified the following predictors of decreased survival: male gender (reference: female), increasing age ≥50 (reference: <40), diagnosis of SQCC, OU, NSCLC NOS and SCLC (reference: ADC), and increasing stage (reference: stage I). (M1 – Table2). Compared to women, the risk of death in men was 38.6% higher (HR = 1.386; 95%CI: 1.295–1.484). The survival probability steadily decreased with age, with a risk of death of 1.354 and 2.454 respectively for groups 50-59 and ≥80. Compared to ADC, except for NSCLC Others, all other subtypes had a higher risk of death: SQCC (+16.7%), NSCLC NOS (+32.9%), SCLC (+37.3%) and OU (+76.4%). However, the independent risk factor with the worst impact in survival was stage, doubling the risk of death with increasing stage: compared to stage I, stage II was 1.8, stage III 3.7 and stage IV 7.8 times higher.
Multivariable Cox regression model to estimate 5-year Hazard Ratios of death (M1).
| HR (95% CI) | p-value | |
|---|---|---|
| Sex | ||
| Female | Reference | |
| Male | 1.386 (1.295–1.484) | <0.0001 |
| Age group | ||
| <40 years | Reference | |
| 40–49 years | 1.299 (0.957–1.764) | 0.094 |
| 50–59 years | 1.354 (1.008–1.821) | 0.044 |
| 60–69 years | 1.529 (1.139–2.052) | 0.005 |
| 70–79 years | 1.853 (1.380–2.488) | <0.0001 |
| ≥80 years | 2.454 (1.815–3.317) | <0.0001 |
| Histology | ||
| NSCLC | ||
| ADC | Reference | |
| SQCC | 1.167 (1.084–1.255) | <0.0001 |
| Others1 | 0.963 (0.838–1.106) | 0.591 |
| NSCLC NOS | 1.329 (1.207–1.464) | <0.0001 |
| SCLC | 1.373 (1.252–1.506) | <0.0001 |
| OU | 1.764 (1.607–1.936) | <0.0001 |
| Stage2,a | ||
| I | Reference | |
| II | 1.838 (1.535–2.202) | <0.0001 |
| III | 3.715 (3.280–4.208) | <0.0001 |
| IV | 7.776 (6.909–8.753) | <0.0001 |
The multivariable model only included cases with disease stage defined (N=6,181)
HR= Hazard Ratio; CI = Confidence Interval; ADC = Adenocarcinoma; SQCC = Squamous cell carcinoma; NSCLC NOS = Non-small cell lung cancer not otherwise specified; SCLC = Small cell lung cancer; OU = Other and unspecified.
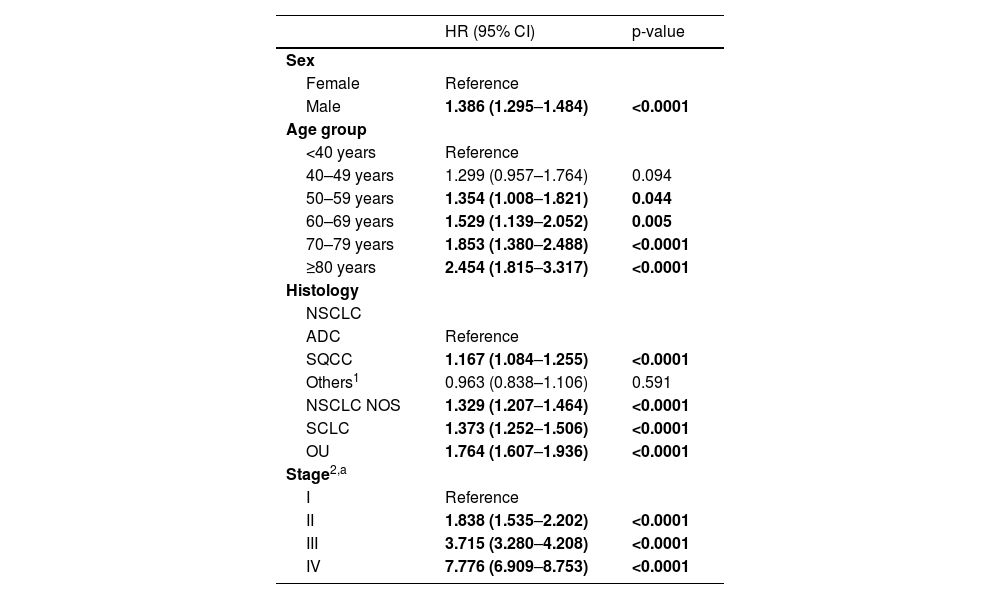
Considering the high proportion of patients missing stage information that were excluded from the multivariable Cox regression models (N= 5,459; 46.9%), we conducted a sensitivity analysis to M1 to understand the differences in HR for the remaining variables with and without the effect of stage. The results showed similar trends by sex and age-groups although somewhat higher HR when stage was excluded. Histology presented more expressive variations between both models, with the higher difference observed for SCLC (+22.9%) in the model excluding stage. Details can be found in Table 3.
Sensitivity analysis to multivariable Cox Regression Model 1 to estimate impact of missing cases related with unknown stage in the Hazard Ratios of death for the remaining variables.
| HR (95%CI) Crude (N=11,640) | p-value | HR (95% CI) Model excluding stage (N=11,640) | p-value | HR (95% CI) Model including stage (N=6,181) | p-value | |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Female | Reference | Reference | Reference | |||
| Male | 1.514 (1.442–1.589) | <0.0001 | 1.427 (1.358–1.500) | <0.0001 | 1.386 (1.295–1.484) | <0.0001 |
| Age group | ||||||
| <40 years | Reference | Reference | Reference | |||
| 40–49 years | 1.875 (1.502–2.341) | <0.0001 | 1.531 (1.226–1.913) | <0.0001 | 1.299 (0.957–1.764) | 0.094 |
| 50–59 years | 1.980 (1.600–2.452) | <0.0001 | 1.524 (1.229–1.889) | <0.0001 | 1.354 (1.008–1.821) | 0.044 |
| 60–69 years | 2.005 (1.622–2.479) | <0.0001 | 1.553 (1.254–2.371) | <0.0001 | 1.529 (1.139–2.052) | 0.005 |
| 70–79 years | 2.461 (1.991–3.043) | <0.0001 | 1.915 (1.547–2.371) | <0.0001 | 1.853 (1.380–2.488) | <0.0001 |
| ≥80 years | 3.538 (2.851–4.391) | <0.0001 | 2.706 (2.177–3.363) | <0.0001 | 2.454 (1.815–3.317) | <0.0001 |
| Histology | ||||||
| NSCLC | ||||||
| ADC | Reference | Reference | Reference | |||
| SQCC | 1.231 (1.169–1.295) | <0.0001 | 1.117 (1.060–1.177) | <0.0001 | 1.167 (1.084–1.255) | <0.0001 |
| Others1 | 0.578 (0.523–0.638) | <0.0001 | 0.620 (0.561–0.685) | <0.0001 | 0.963 (0.838–1.106) | 0.591 |
| NSCLC NOS | 1.603 (1.486–1.728) | <0.0001 | 1.489 (1.380–1.606) | <0.0001 | 1.329 (1.207–1.464) | <0.0001 |
| SCLC | 1.720 (1.609–1.839) | <0.0001 | 1.688 (1.578–1.806) | <0.0001 | 1.373 (1.252–1.506) | <0.0001 |
| OU | 1.756 (1.650–1.869) | <0.0001 | 1.611 (1.513–1.716) | <0.0001 | 1.764 (1.607–1.936) | <0.0001 |
| Stage,2 | ||||||
| I | Reference | n.a. | Reference | |||
| II | 1.968 (1.643–2.356) | <0.0001 | n.a. | 1.838 (1.535–2.202) | <0.0001 | |
| III | 4.077 (3.606–4.609) | <0.0001 | n.a. | 3.715 (3.280–4.208) | <0.0001 | |
| IV | 8.321 (7.406–9.350) | <0.0001 | n.a. | 7.776 (6.909–8.753) | <0.0001 |
46.9% (N=5,459) of cases without disease stage information. Analysis conducted for the remaining 6,181 cases.
HR= Hazard Ratio; CI = Confidence Interval; ADC = Adenocarcinoma; SQCC = Squamous cell carcinoma; NSCLC NOS = Non-small cell lung cancer not otherwise specified; SCLC = Small cell lung cancer; OU = Other and unspecified. n.a. = not applicable
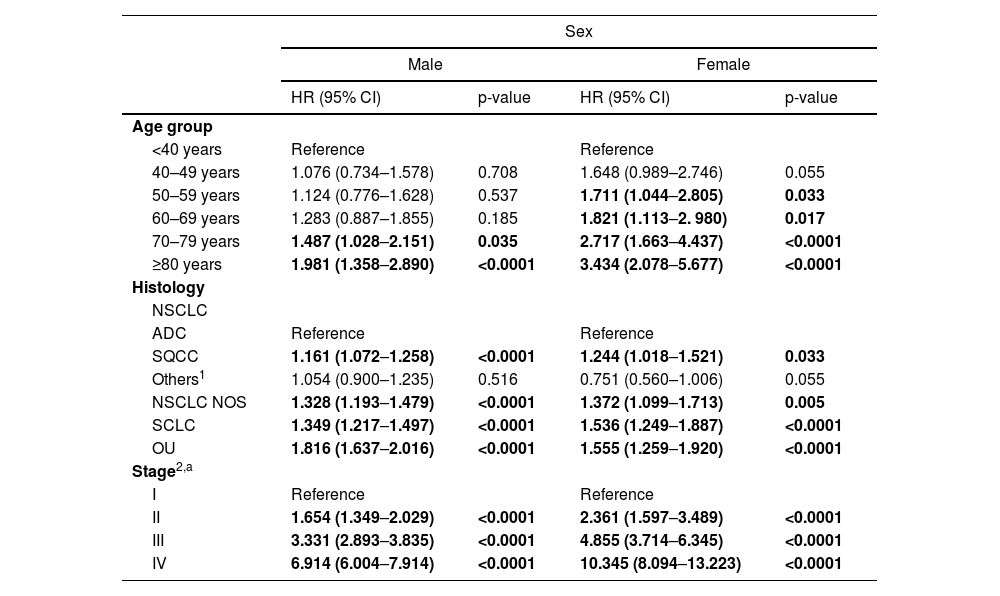
A second Cox regression model stratified by sex identified similar predictors of decreased survival as in M1, except for the age-group above which survival decreased (reference: <40): in males we observed worse survival above 70 while in females was above 50 years (M2 – Table 4). Complete results are included in Table 4.
Multivariable Cox regression model stratified by sex to estimate 5-year Hazard Ratios of death (M 2).
| Sex | ||||
|---|---|---|---|---|
| Male | Female | |||
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age group | ||||
| <40 years | Reference | Reference | ||
| 40–49 years | 1.076 (0.734–1.578) | 0.708 | 1.648 (0.989–2.746) | 0.055 |
| 50–59 years | 1.124 (0.776–1.628) | 0.537 | 1.711 (1.044–2.805) | 0.033 |
| 60–69 years | 1.283 (0.887–1.855) | 0.185 | 1.821 (1.113–2. 980) | 0.017 |
| 70–79 years | 1.487 (1.028–2.151) | 0.035 | 2.717 (1.663–4.437) | <0.0001 |
| ≥80 years | 1.981 (1.358–2.890) | <0.0001 | 3.434 (2.078–5.677) | <0.0001 |
| Histology | ||||
| NSCLC | ||||
| ADC | Reference | Reference | ||
| SQCC | 1.161 (1.072–1.258) | <0.0001 | 1.244 (1.018–1.521) | 0.033 |
| Others1 | 1.054 (0.900–1.235) | 0.516 | 0.751 (0.560–1.006) | 0.055 |
| NSCLC NOS | 1.328 (1.193–1.479) | <0.0001 | 1.372 (1.099–1.713) | 0.005 |
| SCLC | 1.349 (1.217–1.497) | <0.0001 | 1.536 (1.249–1.887) | <0.0001 |
| OU | 1.816 (1.637–2.016) | <0.0001 | 1.555 (1.259–1.920) | <0.0001 |
| Stage2,a | ||||
| I | Reference | Reference | ||
| II | 1.654 (1.349–2.029) | <0.0001 | 2.361 (1.597–3.489) | <0.0001 |
| III | 3.331 (2.893–3.835) | <0.0001 | 4.855 (3.714–6.345) | <0.0001 |
| IV | 6.914 (6.004–7.914) | <0.0001 | 10.345 (8.094–13.223) | <0.0001 |
The multivariable model only included cases with disease stage defined (N=6,181).
HR= Hazard Ratio; CI = Confidence Interval; ADC = Adenocarcinoma; SQCC = Squamous cell carcinoma; NSCLC NOS = Non-small cell lung cancer not otherwise specified; SCLC = Small cell lung cancer; OU = Other and unspecified.
Our study analyzed LC survival across the country stratified by sex, age, stage and histology. In addition, we conducted stratified survival analysis to identify sex differences by age, stage and histology. Our analysis showed a 5-year RS of 15.1% in Portugal, slightly above the average 13% reported in EUROCARE-511,12 but below the 19% reported in the US.13 The high lethality of LC is observed early on, with less than half of the patients surviving at 1-year (42.8%) following diagnosis. Males consistently showed lower levels of survival since year one of follow-up in agreement with literature.25,26
The survival of LC is impacted by age27,28 and a significant decline is observed in 5-year RS between patients <40 and 40-49, from 41.7% to 17.7% and again for elderly ≥80 (7.2%). A similar impact occurs for both sexes but with a more expressive difference observed in the older patients, with a RS in females two times higher (11.6% vs. 4.9%).
Concerning histology, NSCLC Others presents the highest RS in both sexes. Similar findings were not found in the literature, and can be explained by the high proportion of patients in our population diagnosed in early stages (stages I/II 42.6% vs. stages III/IV 57.3%) compared to the average for all histologic subtypes (stages I/II 18% vs. stages III/IV 82%). The same was observed for both sexes, with no significant difference found between NSCLC Others and ADC in Cox regression models. Unsurprisingly, SCLC presented the worst RS (4.3%), considering its known aggressiveness, with rapid disease progression following treatment and early widespread metastasis.29
Stage at diagnosis had the most dramatic impact in survival varying from 66.6% in stage I to 2.4% in stage IV. Although this pattern was previously reported,8,9,30 it highlights the importance of an early diagnosis to improve LC survival.31 The same impact is observed in the sex-stratified results, but with a marked survival advantage in women across all stages. The 5-year RS ratio of female:male is approximately 35% in stage I (80.1% vs. 59.2%) but increases to 2.4 times higher in stage IV (4.3% vs. 1.8%).
In general, women are diagnosed at a younger age, with 12.2% females <50 vs. 7.6% men, diagnosed at earlier stages (19.7% vs. 11.2% in stage I) and more frequently diagnosed with ADC (57.1% vs. 35.8%), which can partially explain the higher survival consistently seen in females.
Globally the differences found between OS and RS were small and reflect the high contribution of LC to early deaths.
In the global multivariable model (M1), aligned with other publications, males have worse survival outcome (HR = 1.386; 95%CI: 1.295–1.484); older age at diagnosis (>50) increasingly predicts lower survival compared with younger patients (<40). Compared to ADC, except NSCLC others, all remaining histologic types have worse survival prognosis.32,33 OU emerges as the most aggressive with HR = 1.764 (95%CI: 1.607–1.936). The worse prognosis of more undifferentiated types has been previously reported, and could potentially be linked with the consequent low accuracy in treatment selection.34
As in most countries, more than 80% of patients were diagnosed with locally advanced and metastatic disease, stages that present a much higher risk of death, respectively 3.7 and 7.8 times compared to stage I, which inevitably contributes to the low LC survival.35 The improvements in treatment that occurred in the last decade with the approval of targeted therapies to checkpoint inhibitors, changed or delayed the fate of patients with metastatic LC; but still, the best solution to improve prognosis would be early detection eventually through screenings in well defined high risk groups.14,36,37
Sex-stratified modeling revealed an earlier impact of age and more pronounced effect of age and stage in the risk of death among females than among males. More advanced stage at diagnosis in men could reflect lower health awareness and higher thresholds for seeking health care.26 Alternatively, observed differences may reflect more aggressive tumor behavior in men, such as faster growth and higher metastatic potential.26,38
However, despite accumulating evidence that sex is one of the most important factors influencing disease risk and response to treatment, the patient's sex is usually not taken into account in clinical decision making. Both men and women might benefit from the development of sex-specific treatment strategies.39
The key strength of our study was the population-based data coverage that gives a comprehensive picture of 5-year LC survival in Portugal, including sex-related differences. The Portuguese population-based cancer registries were created in 1988 requiring all hospitals to collect and provide the data to the regional registry of their geographical area.40
Considering the introduction of new therapeutic options for metastatic disease particularly in the last decade, more recent analyses are needed to address the current survival status for LC in Portugal.
Some limitations need to be mentioned. The population-based registries can have data misclassification or incomplete information, although as they are mandatory the great majority of national data is likely to be included. Additionally, greater completeness of disease stage is needed (N = 5,459; 46.9% missing data). Individual-level data on important risk and prognostic factors is lacking, namely smoking behavior, co-morbidities, mutational biomarkers and performance status, which can be confounders due to their impact on therapeutic options and survival.
Population-based registries must evolve to meet the needs of health care systems, researchers and policy makers.41
Considering the high mortality rate associated with LC and its strong correlation with late stage at diagnosis, there is potential for earlier diagnosis of high-risk individuals through screening with low-dose computed tomography that has shown to reduce mortality.36,37 Identifying adults who meet screening criteria is currently a high public health priority.42
ConclusionsThis study found that the differences between OS and RS in Portugal were small reflecting the high lethality of LC. Male gender and older age are negative prognostic factors for LC survival. Histologic type also plays an important role in survival prognosis and varies depending on gender. Although the study reflects data of a decade ago, and major changes occurred in diagnosis, staging and treatment, particularly for advanced disease, as LC mortality is strongly correlated with late stage diagnosis, all efforts should be made to promote sustainable measures to secure early diagnosis and further improve survival prospects.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author's contributionTeresa Guerreiro was responsible for the concept and writing of the manuscript, data aggregation, analysis and interpretation, Gonçalo Forjaz for concept and revision of the manuscript, data collection and data analysis, Luís Antunes, Joana Bastos, and Alexandra Mayer for data collection and manuscript revision, Pedro Aguiar for data analysis and interpretation, António Araújo for the concept and revision of the manuscript and Carla Nunes for concept of the manuscript, data analysis and interpretation and manuscript revision.
The authors thank all regional population-based cancer registries coordinators for allowing access to the data that enabled this study. The authors thank Diane Cooper, MSLS, for manuscript editing assistance.
The manuscript is original research that has not been published and is not under consideration elsewhere.