Subjects with severe acquired brain injury (sABI) require long-term mechanical ventilation and, as a consequence, the tracheostomy tube stays in place for a long time. In this observational study, we investigated to what extent the identification of late tracheostomy complications by flexible bronchoscopy (FBS) might guide clinicians in the treatment of tracheal lesions throughout the weaning process and lead to successful decannulation.
Subjects and methodsOne hundred and ninety-four subjects with sABI admitted to our rehabilitation unit were enrolled in the study. All subjects received FBS and tracheal lesions were treated either by choosing a more suitable tracheostomy tube, or by laser therapy, or by steroid therapy, or by a combination of the above treatments.
ResultsOverall, 122 subjects (63%) were decannulated successfully. Our subjects received 495 FBSs (2.55 per subject) and as many as 270 late tracheostomy complications were identified. At least one complication was found in 160 subjects (82%). In only 11 subjects, late tracheostomy complications did not respond to the treatment and were the cause of decannulation failure.
ConclusionsIn conclusion, in sABI patients FBS is able to guide successful tracheostomy weaning in the presence of late tracheostomy complications that could get in the way decannulation.
Improved care in the intensive care unit (ICU) has resulted in many patients surviving acute respiratory failure.1 Approximately 10% of critically ill patients receive a tracheostomy to facilitate weaning from prolonged mechanical ventilation support.2 In Italian ICU, 26% of patients maintained tracheostomy despite weaning from mechanical ventilation.3 According to Cox4, 25% of tracheostomy patients died in hospital and 23% were discharged to rehabilitation or long-term care units. When clinical stability is achieved, tracheostomy allows patients to be transferred to other step-down units.2 A multidisciplinary tracheostomy team is recommended to facilitate weaning.5,6
Several decannulation protocols have been suggested in the past, mainly in acute intensive care settings, for subjects whose tracheostomy tube was expected to be removed within a short period of time.7,8-10 In 2014, Santus et al.11 conducted a systematic review of the literature on tracheostomy tube removal to assess predictors of successful decannulation and to propose a predictive score to help clinicians in choosing decannulation timing. Cough effectiveness and the ability to tolerate tracheostomy tube capping were the most widely considered parameters in clinical practice. Indeed, among the 10 reviewed papers, only Ceriana et al.12 considered the absence of tracheal stenosis assessed by endoscopy as a primary requirement for proceeding to decannulation. Hence, in the score proposed by Santus et al.11, airway patency was merely listed among the minor criteria.
Subjects with severe acquired brain injury (sABI), due to trauma, cerebrovascular disease or post-anoxic coma require a prolonged stay in the ICU because of very slow weaning from mechanical ventilation and subsequent difficult decannulation. As motor control and the ability to execute simple voluntary tasks are severely impaired, due to both neurological damage and cognitive disorders,13 cough effectiveness, which is considered a major criterion for successful decannulation11, is not always reliably assessable. The prolonged stay of the tracheostomy tube may cause inflammation and stenosis or excessive cough, and it may also impair swallowing by hindering tracheal elevation against the epiglottis, which is an automatic mechanism to prevent aspiration of food or secretions.8,9 Further, while the incidence of post-tracheostomy tracheal stenosis is around 2.6% in the general population,14 the overall incidence of airway stenosis in subjects with sABI is 20%, a quarter of which were severe; 15% of subjects died as a result of tracheal complications and the incidence of stenosis was significantly higher following tracheostomy than following intubation only.15 Finally, Law16 observed airway lesions in 67% of subjects with a long-term tracheostomy tube, mainly tracheal granuloma (60%) and tracheomalacia (29%). Less frequently observed lesions were tracheal stenosis (14%) and vocal cord and laryngeal dysfunction (8%). Accordingly, the authors concluded by recommending that all decannulation candidates should undergo anatomic examination of the airways. The same recommendation has also been recently highlighted by Enrichi et al.13, who suggested that endoscopic assessment of airway patency also plays a pivotal role for successful decannulation in subjects with sABI.
In this observational study conducted on a large series of subjects with sABI, we investigated to what extent the identification of late tracheostomy complications by bedside flexible bronchoscopy (FBS) might guide clinicians in the treatment of tracheal lesions and, consequently, lead to successful management of the tracheostomy tube throughout the weaning process.
Materials and methodsSubjectsOne hundred and ninety-four subjects were selected among those admitted to the High Intensity Rehabilitation Unit of the Don Gnocchi Foundation, Florence, Italy, from January 2012 to December 2016.
The inclusion criteria were: 1) confirmed diagnosis of sABI; 2) presence of tracheostomy cannula; 3) age > 18 years; 4) stable spontaneous breathing; and 5) absence of fever, sepsis or active infection.
The Institutional Review Board approved the study protocol and all participants, or their legal representatives, signed the informed consent form for personal data processing on admission to the Rehabilitation Units.
Rehabilitation programEach subject underwent a multidisciplinary customised rehabilitation program aimed at recovering neurological and cognitive impairments, at weaning the patient from the tracheostomy tube and at restoring oral feeding. The program was carried out by pulmonologists, neurologists, speech and swallow therapists and physiotherapists. The decision about decannulation was made according as previously described,13 such as tolerance of tracheostomy tube capping for at least 72 h12 and absence of severe dysphagia, defined as the ability to manage secretions, as assessed by the speech and swallow therapist.17,18
Decannulation protocolAccording to previous literature on tracheostomy tube removal,13,17 swallowing assessment was combined with the pathophysiological assessment of respiratory function (airway patency and cough). The protocol included the following preliminary clinical assessments:
- •
arterial O2 saturation > 92% while breathing room air or with oxygen supplementation (if needed), without respiratory acidosis;
- •
evaluation of cough effectiveness, with the assessment of protective cough reflexes;
- •
no significant abnormalities on the chest X-ray;
- •
satisfactory nutritional conditions.
If the above clinical conditions were stable, subjects underwent the following further evaluations:
- •
spontaneous management of oral secretion by the ability to tolerate tracheostomy tube with deflated cuff with stable peripheral O2 saturation and clinical monitoring;
- •
the blue dye test;17
- •
ability to cough and breath by progressively longer capping of the cannula for at least 72 h with no need of aspiration.11
FBS was performed at least once throughout the decannulation process: at the first cannula change and/or while removing the tracheostomy tube and/or when weaning protocol failure occurred, to assess and to manage the reason of failure.
The follow-up until discharge was clinical in all the patients and endoscopic when it was necessary to evaluate the spontaneous healing of milder complications. Most of the non-decannulated patients were transferred to long-term care with the indication to perform replacement of the tracheostomy tube as previously described.19
Airway patency assessmentAirway patency assessment and tube replacement was performed at the subject's bedside by a pulmonologist using the Pentax 15-RBS portable flexible fibrebronchoscope (PENTAX Medical Europe GMBH Hamburg, Germany). Laser therapy was performed with rigid bronchoscopy in acute settings.
FBS was performed through nasal access to assess the supraglottic and subglottic planes, the position and size of the tracheostomy tube and the tracheal patency during quiet breathing, deep breath and cough after cannula removal. When decannulation was not yet possible, during FBS a suitable tube was chosen according to the clinical characteristics of the patients and the size of the trachea to minimize the encumbrance in order to prevent or treat tracheal complications. In general we placed a non-fenestrated tube to minimize friction on the tracheal mucosa, uncuffed tracheal cannula if the management of the oral secretions was good and a tube size that let the air reach the glottic plane.
Data analysisSubjects were divided into two subgroups according to decannulation success or failure. The significance of differences between subgroups was tested using Student's t-test for independent samples, Pearson's chi-squared test and the Wilcoxon rank-sum test for continuous, categorical and ordinal variables, respectively. The analysis of variance was used to assess possible differences in the stay of tracheostomy tube in successfully decannulated patients according to origin. Statistical analysis was performed using the STATA 12 software (Stata Corporation, Collage Station, TX, USA). Statistical significance was set at p < 0.05.
ResultsTable 1 shows the subjects’ clinical characteristics and comparison between decannulated and non-decannulated patients. In 55% of our patients tracheotomy techniques were unknown; 42% of the patients received percutaneous tracheostomy and 3% open surgical tracheostomy.
Subjects’ clinical characteristics and comparison between decannulated and non-decannulated patients.
Data are shown as means ± standard deviation or as absolute numbers with percentages in brackets.
GCS, Glasgow Coma Scale; sABI, severe acquired brain injury; ICU intensive care unit.
(*) From Student's t-test, Pearson's chi-squared test or Wilcoxon rank-sum test, as appropriate.
In our series, 122 subjects out of 194 (63%) were decannulated successfully. Of these, 39 (32%) were decannulated after the first FBS, while the remaining 83 (68%) needed more than one FBS before being decannulated. The mean lapse of time between admission to the rehabilitation unit and decannulation was 59 ± 58 days. The time to decannulation was similar in patients coming from different acute settings: 93 ± 63 days from cardiological ICU, 110 ± 73 days from general ICU, 92 ± 56 days from neurological ICU (p 0.356). None of the decannulated subjects underwent emergency re-cannulation due to lack of airway patency until discharge from rehabilitation.
Successfully decannulated subjects were younger and predominantly male and showed a significantly higher Glasgow Coma Scale (GCS) score on admission to the rehabilitation unit and a significantly shorter stay in the ICU. No significant difference was found regarding the cause of sABI, the lapse of time between tracheostomy and admission to the rehabilitation unit and the number of days spent in the rehabilitation unit.
With regard to the GCS score, 13 out of 122 successfully decannulated subjects (11%) showed on admission a score < 8, which is considered a critical divide;13 however, 8 of them improved their level of consciousness in the course of the rehabilitation up to a GCS score > 10.
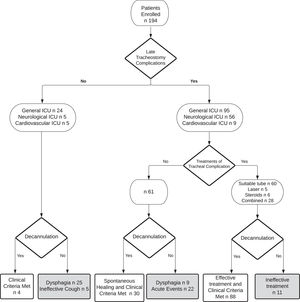
Decannulation was not possible in 72 out of 194 subjects (37%). Among these, the main cause of decannulation failure was severe dysphagia with the inability to manage oral secretions in 34 patients (47%), followed by the occurrence of acute events that prematurely stopped the rehabilitation program in 22 subjects (31%), late tracheostomy complications in 11 subjects (15%) and ineffective cough in 5 subjects (7%) (Fig 1).
Altogether, our 194 subjects received 495 FBSs (2.55 per subject). The observed complications of the bedside procedures were very mild and did not require transferring the patient to an acute setting. The most frequent complication was bleeding in about 15% of procedures: in 10% it spontaneously resolved while in 5% local therapy with tranexamic acid was performed. As many as 270 late tracheostomy complications were identified: 121 granulomas, 103 oedemas, 30 tracheomalacias and 16 tracheal ring ruptures. The late tracheostomy complications, their original settings (neurological, cardiovascular or general ICU), their treatment (tracheostomy tube change, laser, systemic or nebulized steroid therapy or combined therapy) and outcomes are shown in Figure 1. In 9 subjects, tracheal lesions were not treated because they were located between the cannula and the glottis and the presence of concurrent severe dysphagia contraindicated the removal of the cannula. In only 11 subjects, late tracheostomy complications did not respond to the treatment and were the cause of decannulation failure. In these patients, after the rehabilitation discharge, tracheal complications were followed by the acute care ward of origin.
DiscussionIn this observational study, we found a very high incidence of late tracheostomy complications, the vast majority of which could be treated successfully based upon FBS findings, so that we were able to remove the tracheostomy tube in a relevant proportion of subjects and late tracheostomy complications were rarely the cause of decannulation failure.
The aforementioned findings represent the strength of the study. In fact, FBS has been relegated to the role of a minor prediction criterion for successful decannulation.11 However, most of the previous papers7,8-11 tested the criteria for predicting successful decannulation in subjects who had received tracheostomy for any clinical reason, who had been hospitalised in any ward and, mainly, in subjects whose tracheostomy tube had been expected to be removed within a short period of time. This is not the case for subjects with sABI, as they generally require long-term mechanical ventilation; consequently, the tracheostomy tube stays in place for a long time and a high incidence of tracheal lesions is expected. In these subjects, cough effectiveness, which is, undoubtedly, a valuable criterion for decannulation,11 is not always reliably assessable due to the concurrent neurological damages and cognitive disorders that severely impair motor control and the ability to execute simple voluntary tasks.13 In subjects with sABI,13 alongside tracheostomy tube capping, instrumental swallowing assessment and the blue dye test, endoscopic assessment of airway patency also plays a pivotal role in predicting successful decannulation. Our results, not only confirm the high incidence of late tracheostomy complications in subjects with sABI and the relevant role of FBS as an indicator of successful decannulation, but also show that FBS is a valuable tool for guiding clinicians in the management of tracheostomy tubes throughout the weaning process. In fact, the choice of the most suitable tracheostomy tube for every single subject and the targeted medical and/or laser therapy, all of them based upon FBS findings, resolved tracheal lesions and led to successful decannulation in a relevant proportion of subjects.
Another finding of this study was that successfully decannulated subjects showed a significantly higher GCS score on admission to the rehabilitation unit. Although this finding is not surprising, its meaning in clinical practice has not yet been completely clarified and shared. Physicians place greater emphasis on the level of consciousness compared with respiratory therapists who, generally, tend to pay more attention to other factors, such as the cause of respiratory failure and the ability to tolerate tracheostomy tube capping.18,20 Further, Santus et al.11 in their prediction score listed the level of consciousness, classified as drowsy or alert, among the minor criteria for decannulation. Finally, Enrichi et al.13 found that the level of consciousness was not a critical factor for decannulation in subjects with sABI. Within this multifaceted context, our data show that in subjects with sABI, although a good level of consciousness on admission is a positive predictor of successful decannulation in most subjects, a low GCS score on admission may not necessarily be per se a negative predictor because some subjects may improve their level of consciousness in the course of the rehabilitation.
In our series, successfully decannulated subjects showed shorter stay in the intensive care unit. Although this finding is not surprising, it has never been reported before. Most of the previous studies were conducted in acute settings and the number of days spent in the intensive care unit was not considered. Thus, this study adds the notion that in subjects with sABI, the duration of the stay in the ICU is a predictor of decannulation: the shorter the stay, the more likely the decannulation. These results make clear the need to apply early and standardised strategies that reduce the length of stay in intensive care. The early use of non-invasive ventilation21,22 and mechanical cough assist23,24 in patients with prolonged and difficult weaning from invasive ventilation could reduce the stay in ICU, promote early weaning and minimize complications.
Despite the interesting and novel findings of this study, at least two limitations need to be considered. Given the observational design of the study, the lack of a control group represents the main methodological limitation and does not allow us to draw definitive and generalisable conclusions on the management and weaning from tracheostomy tube in patients with sABI. Another limitation is that our study is not multicentre. The general lack of an interventional pulmonologist on the multidisciplinary staff of the ABI rehabilitation unit limits the application of our bedside protocol.
In conclusion, by using FBS we found a very high incidence of late tracheostomy complications, the vast majority of which could be treated successfully based upon FBS findings, so that we were able to remove the tracheostomy tube in a relevant proportion of subjects. In addition, late tracheostomy complications were rarely the cause of decannulation failure. Further, the choice of the most suitable tracheostomy tube for every single subject and the targeted medical and/or laser therapy, all of them based upon FBS findings, resulted in healing tracheal lesions and led to successful weaning from the tracheostomy tube even in the relevant proportion of subjects who did not fulfill the decannulation criteria on the first clinical and FBS assessment. Thus, the take-home message from this study is that FBS is strongly recommended to guide clinicians throughout the weaning process and to achieve successful decannulation in subjects with sABI and, probably, in all subjects with long-term stay of the tracheostomy tube.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.