In this review, we present the effects of lung hyperinflation on the cardiovascular system (CVS) and the beneficial outcomes of different deflation treatment modalities. We discuss the effects of long-acting bronchodilator drugs, medical and surgical lung volume reduction on the performance of the CVS.
Although there is a small number of studies investigating lung deflation and the CVS, the short-term improvement in heart function was clearly demonstrated.
However, more studies, with longer duration, are needed to verify these significant beneficial effects of deflation of the lungs on the CVS. Dynamic hyperinflation during exercise could be a research model to investigate further the effects of lung hyperinflation and/or deflation on the CVS.
It is well known, that the respiratory and cardiovascular systems are strongly interconnected in health and disease.1 Each disease, cardiovascular or respiratory, impacts negatively on morbidity and mortality of the other,2 and both have common risk factors, such as smoking. A classic example is chronic obstructive pulmonary disease (COPD) where the most frequent co-morbidities are cardiovascular.3,4 Similarly, patients with cardiovascular diseases, especially ischemic, usually demonstrate signs of respiratory impairment, which may need to be investigated by pulmonary function tests (spirometry).5-7
The main mechanical defect in the most common chronic lung diseases (COPD and asthma) is lung hyperinflation (LH). However, the effects of LH on the cardiovascular system (CVS) have not been adequately studied. We reviewed the literature using PubMed, (1971–2022, keywords: lung hyperinflation, heart, cardiovascular) and found 108 reports from which 71 were relevant.
Lung hyperinflation and the respiratory systemPatients with airway diseases often have symptoms mainly caused by lung hyperinflation.8,9 This can occur in stable disease but most crucially during an exacerbation.10 The cardinal effects of LH on the respiratory system are a reduction in respiratory muscles efficiency, as pressure generators and a significant increase in the work of breathing.11 Hyperinflation puts the respiratory muscles in a disadvantaged position to produce pressure by affecting their length, curvature, and blood supply.11 These can lead to respiratory muscle fatigue and failure. In addition, LH reduces the area of opposition, the fulcrum of the respiratory muscles further reducing their efficiency. As a result, a reduction in inspiratory capacity (IC) impairs the ability to increase ventilation when needed.12 Due to these effects on the respiratory muscles, the vital breathing pump, hyperinflation can cause ventilator failure and death.13,14These events are noted in both COPD and asthma.
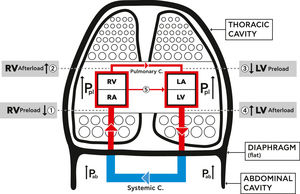
Lung hyperinflation and the cardiovascular systemLung hyperinflation affects the function of the CVS by reducing venous blood return, increasing pulmonary vascular resistance, and compressing the intra-thoracic cardiovascular parts.15-21 These mechanisms are summarized in Fig. 1.1.
Schematic diagram showing the mechanical effects of lung hyperinflation on the cardiovascular system (CVS). Hyperinflation increases intra thoracic pressure (Pleural pressure (Ppl)), the pressure on the parts of the CVS inside the thoracic cavity, increases abdominal pressure (Pab) and flattens the diaphragm.
1. RV Preload reduced: decreased venous blood return due to venous compression {Ppl +Pab+ flat diaphragm) and increase RA pressure.
2. RV Afterload increased: increased RV end-diastolic and pleural pressures, and increased resistance of pulmonary circulation.
3. LV Preload reduced: decreased filling pressure and compliance of LV plus cardiac fossa's external compression (Ppl).
4. LV Afterload increased: increased LV trans -mural and abdominal pressures.
5. Increased ventricular interdependence and leftward shift of septum.
{Pulmonary C.=circulation, Systemic C.=circulation, RA=Right Atrium, RV= Right Ventricle, LA= Left Atrium, LV= Left Ventricle, Ppl= Plural pressure, Pab= Abdominal pressure}.
LH significantly increases the intra-thoracic and abdominal pressure and flattens the diaphragm, thus leading to a significant reduction of venous blood return from the abdominal to the thoracic cavity due to vein compression. This in turn causes a reduction in the right ventricular filling and in its preload (Fig. 1.1).16
In pulmonary diseases, the distraction of lung parenchyma and its vasculature leads to pulmonary hypertension and increased pulmonary vascular resistance, termed “ core pulmonale”.22 In addition, the increased pleural pressure on the pulmonary capillary bed increases RV afterload (Fig. 1.2). This leads to a reduction in left ventricular (LV) compliance and external compression, finally causing a reduction in the LV preload (Fig. 1.3). Additionally, the increased abdominal pressure leads to an increase in LV afterload (Fig. 1.4).23-27 A left sided shift of the septum and increased ventricular interdependence also impair the cardiac function (Fig. 1.5).15-18,20,21 The above mechanisms cause a significant reduction in LV stroke volume and total cardiac output28-30 and impair the oxygen delivery to the tissues, including the myocardium. Hyperinflation, especially at a dynamic stage, increases, even more, the already positive intrathoracic pressure causing augmentation of the external mechanical compression of the heart.17 This affects the right and left ventricular filling during the diastolic phase and impairs the myocardial function during systole. It thus, increases even further the LV afterload by increasing its transmural pressure.23,24,26-28
This reduced cardiovascular function is seen even in stable static LH but increases dramatically during an acute exacerbation contributing to unfavorable outcomes. Studies demonstrate that dynamic hyperinflation leads to reduction in the overall intrathoracic blood volume, LV end-diastolic volume, stroke volume and total cardiac output.20,28,29 The significant consequence is reduction of oxygen delivery to vital tissues such as myocardium and respiratory muscles, contributing to cardiac and respiratory failure and overall mortality.
Treatment of lung hyperinflationGiven the implication of hyperinflation on the lungs and the heart it becomes obvious that treating hyperinflation is of paramount importance. The management of LH includes pharmacological and interventional treatments (medical or surgical lung volume reduction).
Long-acting bronchodilator medications, such as muscarinic and b2 –long-acting agonists have shown to significantly reduce hyperinflation.30-36 A systematic metanalysis run by Di Marco et al. showed that long-acting bronchodilators improve exercise capacity and hyperinflation in COPD patients.37 Their deflating effect is greater than their Broncho dilating function, since IC, the index of hyperinflation, improves to a greater degree than FEV1, the classical index of bronchoconstriction.30 This particular effect led to a suggestion to rename them from bronchodilators to deflators.38
Lung volume reduction surgery had been shown to reduce hyperinflation in well-selected emphysematous patients.39 Similarly medical lung volume reduction via fiber optic bronchoscopy also improves lung mechanics by deflating the lungs.40 Although these treatments have been proven to reduce hyperinflation their cardiovascular implications have not been adequately studied.41
Pharmacological lung deflation and the CVS functionOne of the first studies to suggest that bronchodilator drugs affect heart function was reported by Travers et al. in 2007 which showed an improvement of cardiovascular response to exercise in patients receiving tiotropium.42 The study concluded that tiotropium may be beneficial to cardiac function during exercise through mechanical unloading on the ventilator muscles.
Similarly Laveneziana et al. reported that bronchodilators improve heart rate during exercise.43 Santus P et al. in 2015, showed that indacaterol improves the right ventricular compliance indexes by deflating the lungs.44In 2016 Stone et al. in a randomized- placebo, crossover-controlled study investigated CVS structure and function after lung deflation using magnetic resonance techniques in COPD patients. Use of fluticasone Fluroate and Vilanterol achieved a reduction in inspiratory capacity and residual volume versus placebo. In addition, they reported a 5.8 ml/m2 increase in right ventricular EDVI, 3.63 ml/m2 in left ventricular EDVI and 2.33 ml/m2 in left atrial EDSVI.45 RV ejection fraction remained stable but RV stroke volume and pulmonary artery pulsatilily increased. The authors concluded that pharmacological treatment by lung deflation had consistent beneficial effects on cardiac function and pulmonary vasculature. Although, this was a very short study of only 14 days, Watz H, in an editorial, claimed that deflation of the lungs by Pulmonologists is beneficial for the heart.46
More recently Hohlfeld et al. studied the effects of lung deflation using indacaterol plus glycopyronium on ventricular filling in a double-blind, randomized, cross-over, placebo- controlled study.47They showed that dual Broncho dilation significantly reduces lung hyperinflation. In addition, left and right ventricular end diastolic indexes, stroke volume and left ventricular end-systolic volume index increased versus placebo. Heart rate, LV and RV ejection fraction and cardiac mass did not change. The authors reported that, mechanistically, the increase in left ventricular end-diastolic volume with sustained ejection fraction and cardiac mass supports the notion that lung deflation increases LV and RV pre-load, leading to an adaptive increase in cardiac output according to Frank- Sterling mechanism. The authors concluded that LABA-LABA combination positively affects the function of the heart given the known cardiovascular impairment in COPD. In a review Struß et al. concluded that dual long -acting bronchodilator drugs improved the detrimental lung-heart imbalance due to hyperinflation and similar but less pronounced effects were reported with single bronchodilator therapy.48
In an observational study, maintenance therapy, especially with LABA-LAMA, was linked to changes in left atrium size, in accordance with the previous short -term interventional trials and concluded that maintenance treatment could also have a beneficial impact on CVS in COPD.49 A meta-analysis by Zhang et al. showed that combination indacaterol and glycopyrronium improved not only the lung function but also the cardiovascular events.50 The studies by O'Donnell and others have shown that dynamic in addition to static hyperinflation, induced by exercise, could be a research model to investigate the effects of the “deflator” medications on the CVS. Moreover, the deflation effects on CVS could be studied in detail during and after acute exacerbations in COPD patients.35,37,43,51–54
Lung volume reduction surgery (LVRS) and the CVSSeveral studies including the pivotal NETT study have showed that LVRS is an effective method to improve pulmonary function in severe emphysematous patient.55-57
However, the reports of the effects of LVRS on cardiovascular function are inconsistent.58-61 Some studies show improvement in RV function after surgery,60 decreased pulmonary capillary wedge pressure55 reduced respiratory variation in pulmonary arterial diastolic pressure as well as increased RV and LV filling volumes.61 In contrast, there are reports of increased resting pulmonary arterial systolic pressure and pulmonary vascular resistance.18,58,59,61
Recent studies showed that pulmonary hypertension and pulmonary arterial pressure did not change significantly after surgery and pulmonary capillary wedge pressure decreased at end-expiratory level. This may be an indication that LVRS reduces intra-cardiac pressures by a decrease in intra-thoracic pressure. However, those effects are minimal and thus, the beneficial outcomes of LVRS on severe emphysema are predominantly due to the effects on respiratory mechanics.18,57-61 It is likely that the controversial data are the result of the timing of the surgery; performed often too late in the course of the disease. Therefore, more studies are needed to clarify the implication of LVRS to the CVS.
Medical lung volume reduction (MLVR) and the CVSSimilarly, to LVRS, bronchoscopic MLVR significantly reduces lung hyperinflation.62-69 However, very few and small studies have investigated the effects of MLVR on cardiovascular function. Pizarro et al. investigated the extent of RV functional changes following MLVR using speckle tracing-based RV deformation analysis and showed beneficial effects on RV contractility after MLVR.62
In a small study Eberhardt et al., using right heart catheterization found a decrease in mean pulmonary artery pressure and in pulmonary capillary wedge pressure as well as an improvement in cardiac index, 90 days after MLVR(68). More recently, van der Molen evaluated the cardiac pre-load, RV end-diastolic volume index, cardiac output, myocardial contractility, and pulmonary artery pressure before and after MLVR using ERV.70 They reported a significant increase in right ventricle EDVI, improvement in LV stroke volume, cardiac output, and ventricular contractility. Although blood flow within the pulmonary artery expanded there was no increase in pulmonary pressure.70,71
All the above studies agree that MLVR acts beneficially on the heart and thus demonstrate how the Pulmonologist can improve heart mechanics. However, more studies with a larger number of subjects are needed to verify these results.
Summary- conclusionsThis review summarizes the available evidence supporting the argument that lung deflation not only improves lung mechanics by reducing the work of breathing, but also positively affects the heart and the pulmonary circulation. These benefits are more evident in medical (pharmacological and bronchoscopical) interventions than in surgical ones (surgical volume reduction). However, larger studies are needed, of longer duration, to clarify not only the acute effect of lung deflation on the heart but also the long-term clinical relevance of those effects on morbidity and mortality. Dynamic, in addition to static, hyperinflation during exercise is a valuable research tool to investigate the effects of hyperinflation and /or deflation on the cardiovascular system. Moreover, studies needed to investigate deflation and heart function, during and after an acute exacerbation of COPD. Finally, although the beneficial effects of deflation have been studied in short duration trials it can be stated that by deflating the lungs Pulmonologists help the Cardiologist!