Severe asthma can be defined as an “asthma which requires treatment with high-dose inhaled corticosteroids (ICS) + LABA to prevent it from becoming ‘uncontrolled’ or which remains ‘uncontrolled’ despite this therapy”.1 It is an important public health problem strongly associated with a significant health-related quality of life (HRQL) burden 2 and with considerable healthcare costs, almost twice those of non-severe asthmatics.3
Several studies have assessed the factors associated with HRQL in severe asthmatics, demonstrating the key role played by asthma control and comorbidities.2 However, very few studies have assessed the impact of detailed respiratory symptoms on HRQL. A recent study conducted by Louis et al.4 has demonstrated that dyspnoea was the most impactful symptom on the lives of mild asthmatics. However, to the best of our knowledge, no study has ever assessed the impact of specific respiratory symptoms on HRQL in a population of severe asthmatics. Knowing which symptoms are the most impactful on the lives of severe asthmatics is useful for adopting personalised care strategies.
A cross-sectional study was conducted between 2018 and 2022 on a population of T2 high severe adult asthmatics recruited from the Liege University Hospital Asthma Clinic (Belgium) prior to initiation of biologics (n = 143). All had had at least two exacerbations in the 12 months prior to the visit, with baseline FEV1<80 % predicted. T2 high profile was defined as either one of the following characteristics: (i) blood eosinophil count>300/µl, (ii) FeNO>25 ppb, (iii) IgE>76 KU/l with sensitisation to at least one perennial allergen. Lung function testing was performed by spirometry (Spiro bank; MIR, Rome, Italy) to measure expiratory flow rates. FeNO was measured at a flow rate of 50 ml/s (NIOX; Aerocrine, Solna, Sweden) before spirometry. Asthma control was measured using the Asthma Control Test (ACT).5 Symptoms were measured by five-point Likert scales ranging from 1 to 5 (5 expressed the highest intensity). HRQL was measured by the mini asthma quality of life questionnaire (AQLQ) composed of 15 items scored on a seven-point Likert scale ranging from 1 to 7 (7 expressed the highest QOL).6 Median and interquartile range (IQR) were used to describe quantitative variables, while percentages and counts were used for qualitative variables. The associations between symptoms intensity and AQLQ were determined using the Spearman correlation coefficient. Multiple linear regression analyses were performed to identify symptoms independently associated with AQLQ. All statistical analyses were performed using GraphPad Prism software (version 9.4.1) at a significance level of 0.05.
The median age of our patients was 55 (41–65) years and 64 %(92) were female. 61 %(87) were never-smokers, 27 %(39) ex-smokers and 12 %(17) current smokers. The median baseline FEV1 was 71 %(47 %−82 %) predicted and the FEV1/FVC was 74 %(61 %−79 %).
The median global mini-AQLQ was 3.9 (3–5.1) and the median ACT was 12 (9–17)(17 % patients with controlled asthma as shown by ACT≥20). The median values of cough, airway secretion, chest tightness, dyspnoea and wheezing intensity scales were 3(2–4), 3(2–4), 3(2–4), 4(3–4) and 3(2–4), respectively.
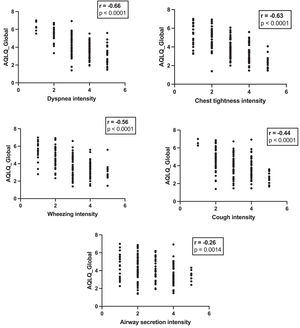
For each symptom, a higher intensity score was significantly correlated with low global AQLQ. However, different Spearman correlation coefficients were found depending on the symptoms. Dyspnoea had the strongest correlation with global AQLQ (rs=−0.66; p<0.0001), while airway secretion displayed the weakest correlation (rs=−0.26; p = 0.0014) (Fig. 1). After adjusting for age, sex, BMI, FEV1% predicted, FEV1/FVC% and asthma control, multiple linear regression analysis (MLRA) showed that only dyspnoea was significantly associated with global AQLQ (Table 1). MLRA also revealed that dyspnoea was independently associated with the activity dimension, while airway secretion and cough were associated with the emotive dimension (Table 1). In addition, cough emerged as an independent predictor of the environmental dimension (Table 1).
Multiple linear regression for the relationship between symptom intensity scales and global AQLQ and its 4 dimensions (n = 143).
Abbreviations: AQLQ, asthma quality of life questionnaire; ACT, asthma control test; BMI, body mass index; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity.; *p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001.
In this study, dyspnoea was the only symptom significantly associated with global AQLQ. It is in line with our recent study4 focusing on mild asthmatics. Whatever the level of severity of the disease, an increase in dyspnoea is a sign that the patient's quality of life is deteriorating, necessitating appropriate healthcare measures. It would be interesting to follow these same patients longitudinally to determine the effect of biologics treatment on the level of respiratory symptoms’ intensity. Even if a recent qualitative study demonstrated the revolutionary effect of biologics on the lives of severe asthmatics,7 drug impact would be enhanced if part of a global approach based on the patient's needs and expectations, as reported through patient-reported outcome measures (PROMs). It is the combination of a precision medicine based on biologics with a PRO-cision medicine 8 that will undoubtedly contribute to improving the health and quality of life of severe asthmatics, as well as the quality and safety of care.
In conclusion, dyspnoea is the most impactful symptom on the lives of severe asthmatics, even if other symptoms - airway hypersecretion and cough - appear to influence independently some of the dimensions of quality of life.