The use of medical masks and respirators as personal protective equipment is pivotal to reducing the level of biological hazard to which healthcare workers are exposed during the outbreak of highly diffusible pathogens, such as the recent novel coronavirus SARS-CoV-2. Unfortunately, during this pandemic, supplies are rapidly running out worldwide, with potential consequences for the rate of occupational infections. Also, knowledge about specific characteristics of respirators is of utmost importance to select the proper type according to the clinical setting. A wide variety of literature is available on the topic, but mostly based on Influenza viruses infection models. Clinical evidence on the use of respirators is poor and interest in the topic has not been constant over time. A better understanding of SARS-CoV-2 transmission is needed, together with high-quality clinical data on the use of respirators or alternative devices. Moreover, healthcare workers, regardless of their level of experience, should receive specific training. This review aims to summarize the available evidence on the use of medical masks and respirators in the context of viral infections, especially the current coronavirus disease 2019 (COVID-19).
The outbreak of highly diffusible pathogens, such as the recent pandemic of SARS-CoV-2 infection, can increase the level of biological hazard to which healthcare workers are exposed thus requiring the use of personal protective equipment (PPE). Healthcare institutions should also plan the early isolation of sources and provide training program on the appropriate use of PPE.1 PPE, defined as ‘equipment worn to minimize exposure to hazards that cause serious workplace injuries and illnesses’, includes masks and respirators.2 The use of PPE and the application of other safety measures, especially in the context of a public health emergency of international concern, is regulated by international and national authorities that issue indications for healthcare workers and general population, according to the characteristics of transmission and the different levels of exposure to risk.3 Unfortunately, in the case of a pandemic, the supply of PPE can be insufficient or heterogeneously distributed around the world, due to centralized production hubs, transport difficulties, lack of stockpiles, panic buying and appropriate heavy use.
Medical mask and respirators
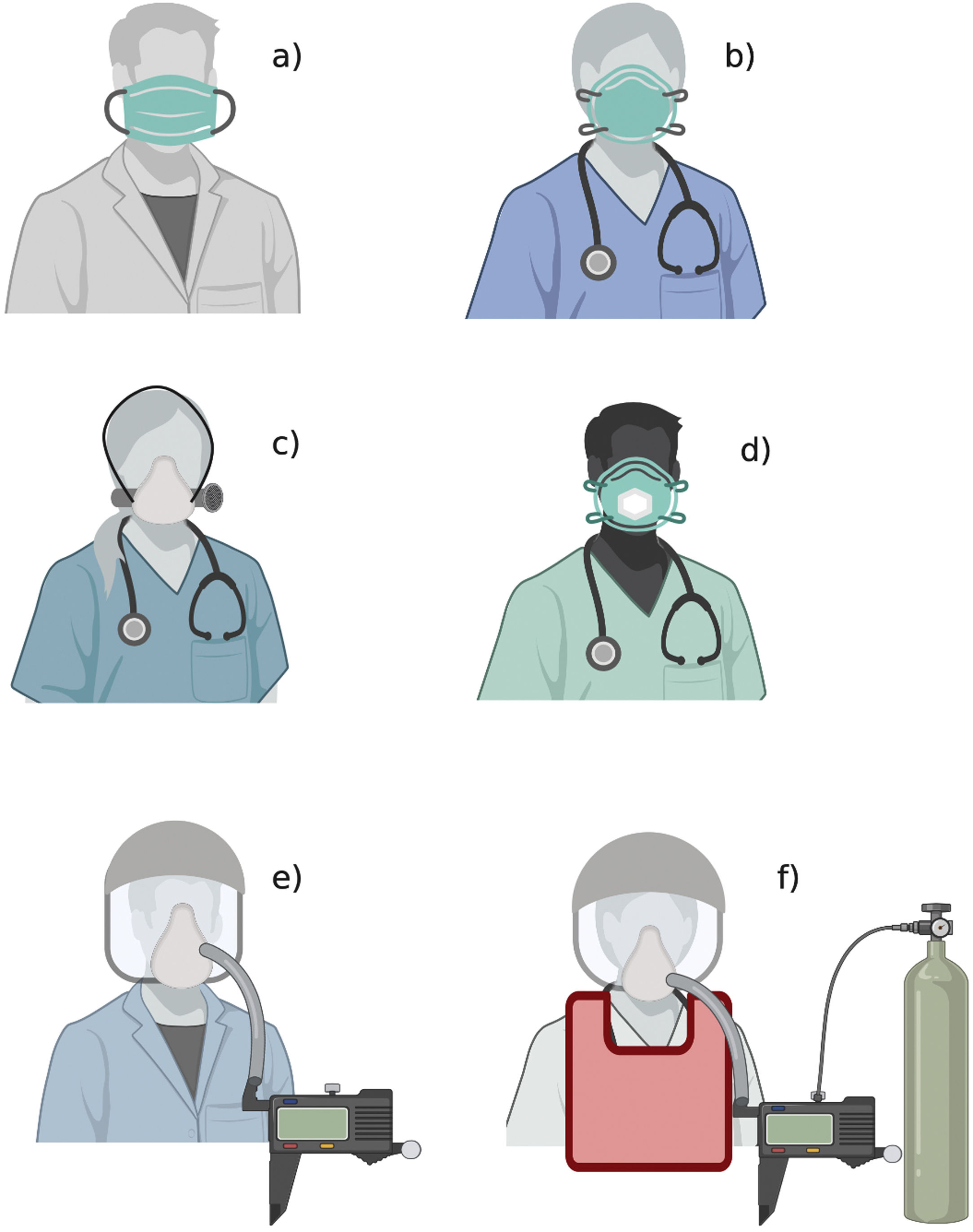
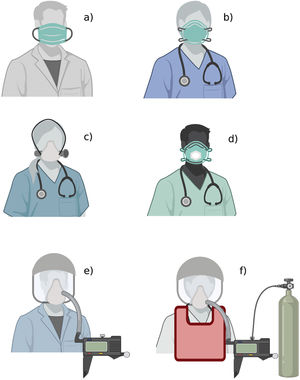
The figure shows the available types of medical masks and respirators: a) medical mask; b) filtering facepiece respirator; c) elastomeric respirator; d) filtering facepiece respirator with expiratory valve; e) powered and supplied air respirator; f) atmosphere-supplying respirator. The figure does not show other PPE elements (gloves, gown, goggles, face shield, boots).
RNA viruses, such as SARS-CoV-2, SARS-CoV-1, Ebola and MERS virus, are examples of pathogens causing infectious diseases with zoonotic origins and human-to-human transmission, where spread can be restricted by the appropriate use of PPE. To date, the transmission of SARS-CoV-2 is reasonably supposed to be mediated by respiratory droplets and contact routes.4,5 Recommendations about the appropriate use of PPE have been controversial and at times conflicting,6 and continuous updates have been released during the current SARS-CoV-2 pandemic,7 with a rising demand for incontrovertible and clear data.
The importance of the availability of an adequate supply of PPE and the training level of healthcare workers in its correct use in the healthcare setting has been evident during the current Coronavirus diseases 2019 (COVID-19) since a high proportion of healthcare workers have been infected. 8,9
The aim of this review is to summarize the available evidence on the use of medical masks and respirators in the context of viral infections, with a specific focus on COVID-19.
MethodsSearch strategyFor the purpose of this review, we searched PubMed, EMBASE, and MEDLINE for pre-clinical and clinical studies on the use of medical masks or respirators in the context of viral infections up to 3 April 2020. Our search included the keywords ‘mask’, ‘respirator’, ‘ffp’, ‘droplet’, ‘aerosol’, ‘coronavirus’ and ‘viral infection’ as exact phrases and also a combination of broad subject headings according to databases syntax. After exclusion of duplicates and abstracts, two authors (MI, AC) independently screened full-text papers to include the most relevant on the topic. Snowballing search on the references of selected articles was also performed.
Medical masks and respiratorsPersonal protective equipment includes medical masks or respirators, used to protect the wearer from droplets, airborne particles and body fluids potentially contaminating the face. 10,11
The term ‘respirators’, in the context of personal protective equipment, should be intended as filtering media, usually in the form of half-face or full-face masks, used as protection for healthcare workers exposed to pathogens.
Types of device and characteristicsThe main performance characteristics of medical masks and respirators are summarized in Table 1.
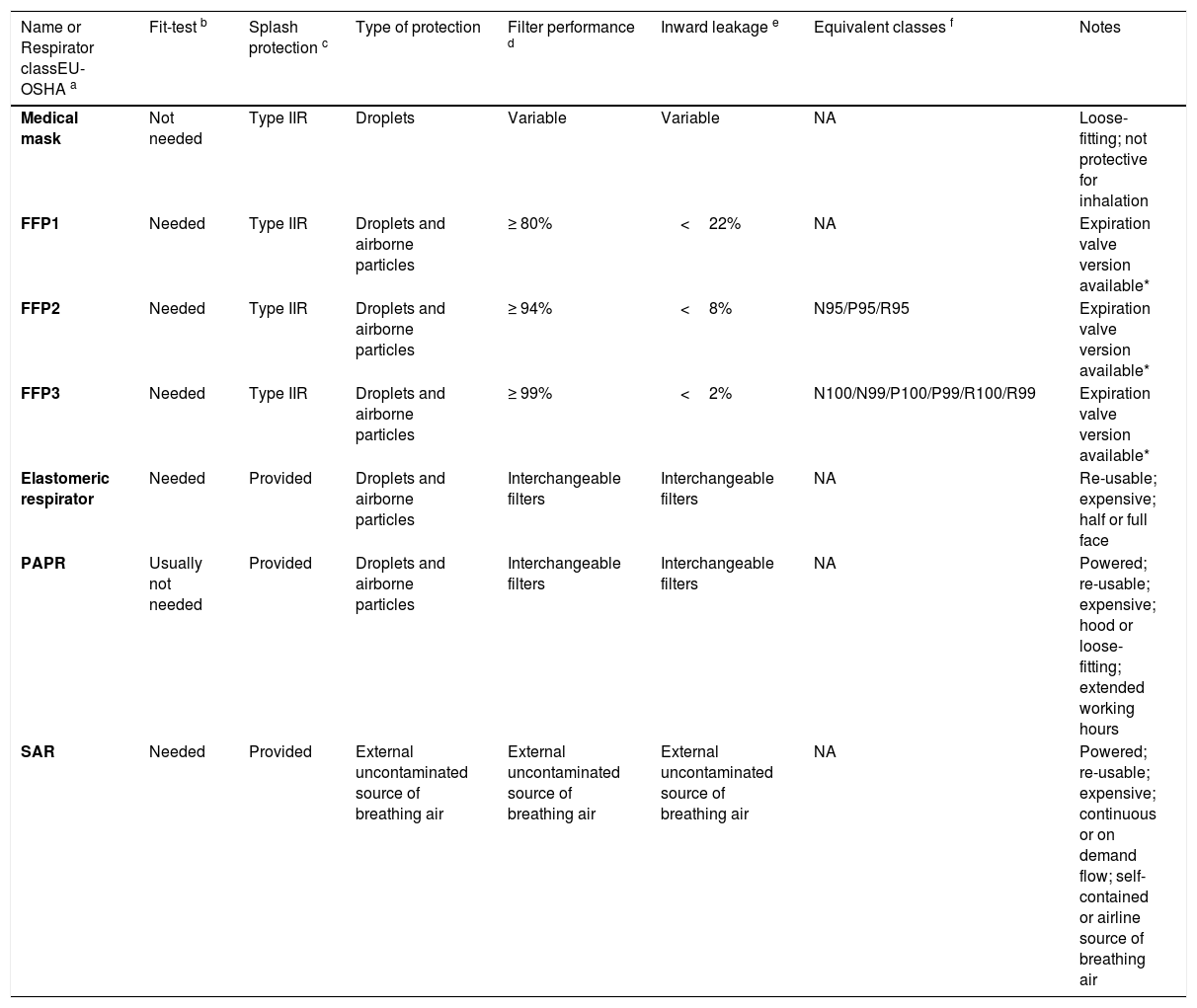
Characteristics of surgical masks and respirators.
| Name or Respirator classEU-OSHA a | Fit-test b | Splash protection c | Type of protection | Filter performance d | Inward leakage e | Equivalent classes f | Notes |
|---|---|---|---|---|---|---|---|
| Medical mask | Not needed | Type IIR | Droplets | Variable | Variable | NA | Loose-fitting; not protective for inhalation |
| FFP1 | Needed | Type IIR | Droplets and airborne particles | ≥ 80% | <22% | NA | Expiration valve version available* |
| FFP2 | Needed | Type IIR | Droplets and airborne particles | ≥ 94% | <8% | N95/P95/R95 | Expiration valve version available* |
| FFP3 | Needed | Type IIR | Droplets and airborne particles | ≥ 99% | <2% | N100/N99/P100/P99/R100/R99 | Expiration valve version available* |
| Elastomeric respirator | Needed | Provided | Droplets and airborne particles | Interchangeable filters | Interchangeable filters | NA | Re-usable; expensive; half or full face |
| PAPR | Usually not needed | Provided | Droplets and airborne particles | Interchangeable filters | Interchangeable filters | NA | Powered; re-usable; expensive; hood or loose-fitting; extended working hours |
| SAR | Needed | Provided | External uncontaminated source of breathing air | External uncontaminated source of breathing air | External uncontaminated source of breathing air | NA | Powered; re-usable; expensive; continuous or on demand flow; self-contained or airline source of breathing air |
The table provides a summary of the main characteristics of medical masks and respirators.
Data were retrieved from ECDC and OSHA documents. [10,11]
The common name of the device or the respirator class, according to EU-OSHA classification, is reported.
A fit test with an indicator aerosol should be performed before first use of a model/size. If the test is positive, the respirator is leaking, and another model or size must be chosen.
Protection from body fluid splashes. If the device is not certified as splash-proof, a separate hood should be used for this purpose.
Filter performance measures the reduction in concentration of specific test aerosols passing through the filter. It is calculated at specific standard conditions that can vary according to national regulations. Minimal variations can occur among equivalent classes around the world.
Inward leakage measures the amount (%) of a specific aerosol allowed to enter the device in a test chamber.
Respirators performance characteristics are tested at national regulatory standard conditions. These standards have similarities around the world; thus recommendations usually refer to a specific class and its foreign equivalent models. Examples of FFP2 equivalents: N95 (United States), KN95 (China), P2 (Australia/New Zeland), DS (Japan), Korea 1st class (Korea).
The presence of an expiratory valve results in a more comfortable breathing, offering less resistance to exhalation. The valve also reduces goggles fogging. Valved respirators are usually not certified as splash-proof.
EU-OSHA: European Agency for Safety and Health at Work; FFP: Filtering facepiece; NA: not available; N-: tested with NaCl filter loading; R-: tested with dioctylphthalate filter loading; P-: tested with dioctylphthalate at maximum filter degradation; PAPR: Powered Air-purifying respirator; SAR: Atmosphere-supplying respirator
Medical masks are loose-fitting and disposable. They are meant to reduce the spread of the wearers’ respiratory droplets to other people and the environment and to provide a general protection of the wearer from large droplets, usually generated by cough or sneezing, and body fluid splashes. Type I medical masks are generally used for patients with the aim of controlling the source, and Type II or Type IIR by healthcare workers in operatory room or procedural settings. The main difference among the types is according to their bacterial filtration efficiency, i.e. the efficiency as a barrier to bacteria penetration. The protection from splashes is only provided by Type IIR medical masks, where R stands for ‘resistant’. 10–12
Filtering facepieces respirators (FFR) are tight-fitting and disposable protective devices, designed to filter airborne droplet nuclei (defined as non-oil based particles<5μm in diameter 13) they are registered as inhalational protective devices. They are differently labelled on the basis of their filtration properties and of the national regulations defining the standard conditions they were tested at.14 As an example, the European label ‘FFP2’ refers to a respirator able to reduce a specific aerosol concentration of at least 94%, while ‘FFP3’ corresponds to a filtration performance of at least 99%. National regulatory standards have similarities around the world and recommendations usually refer to a specific class and its foreign equivalent models. Examples of FFP2 equivalents are N95 (United States), KN95 (China), P2 (Australia/New Zealand), DS (Japan) and Korea 1st class (Korea).
Worth noting, FFR are tested at a high flow-rate (85-95 L/min) and with test aerosol of around 0.3-0.5μm in diameter. As a consequence, their real-life performances should be better than the tested one. Filtering performance strongly depends on fitting, and healthcare workers should test different devices to find the best fitting model and size for their face. Filtering face-piece respirators are not intended to be one-size-fits-all. For example, the presence of a beard can alter the sealing, and shaving is required. A fit test with an indicator aerosol should be used to evaluate the presence of leakages and to confirm the choice of the respirator model and size. Inhalation and exhalation seal checks will be then performed before every use, to tighten the device properly and confirm that it was put on properly. Filtering facepiece respirators are also available in versions with an expiratory valve, which make them more comfortable for long-time wearing. The valve, in fact, opens during the expiratory phase of the wearer's breathing, allowing the exhaled air to flow-out. The protection from body fluids and splashes is rarely guaranteed by valved FFR, and has to be confirmed by the label “Type IIR”, as for medical masks. The presence of an expiratory valve also reduces goggles fogging.10,11 The nominal protection factor is an important index of a respirator performance. It is measured as the ratio between external concentration of contaminant and its concentration measured on the inner side of the device (Cout/Cin). If we assume Cout to be 1, with Cin=(1 - filtration performance), we can easily calculate the nominal protection factor. As an example, a respirator with a 94% filtration performance, will have a nominal protection factor of 16. In this case, the value means that the contaminant is 16 times less concentrated inside the device than in the external environment. Another parameter to be known is the threshold limit value, a threshold level of concentration, specific for each contaminant, which must not be exceeded if the safety of the wearer is to be guaranteed. The real-life protection given by a respirator, in fact, depends on its assigned protection factor, an index that depends on the protection factor provided by the respirator, but also by the ratio between the concentration of the contaminant and its threshold limit value.11 In the case of some biological contaminants, such as the case of SARS-CoV-2, a threshold limit value is not known, and so the assigned protection factor of the respirator remains unknown. However, a reasonable estimate of assigned protection factor, intended as real-life protection guaranteed by the device, can be given by the respirator protection factor or by its filtration performance, if other measures are used to minimize Cout to the farthest level from the threshold limit value. Thus, especially in the context of an unknown contaminant, these concepts and common sense make clear the importance of a safety program including different measures (use of PPE, room ventilation, social distancing, source control).
In the cases of insufficient supplies or if required for specific characteristics of contaminant, disposable FFR can be substituted by alternative types of respirators, not usually common in the healthcare settings, such as elastomeric respirators and powered air-supplying respirators. Atmosphere-supplying respirators are intended for particularly hostile environments.
The elastomeric respirators are re-usable devices, made of synthetic materials that can be disinfected and have interchangeable filters. 10,11
The powered and supplied air respirators are battery-powered reusable respirators composed of hoods or loose-fitting masks, provided with interchangeable and disposable filters, such as high-efficiency particulate air filters. An essential part of the correct use of re-usable PPE, typically used in labs setting, is an accurate cleaning/disinfection procedure. Another device is the atmosphere-supplying respirator, which delivers air to the wearer from an external uncontaminated source. This feature becomes relevant in highly toxic environments or in the case of oxygen deficit. Concerns about the clinical use of re-usable PPE are: difficult communication, due to the noise generated by the power-unit, the need for electricity or batteries and the exposure of personnel in charge of disinfection to an additional biological risk.10,11,15 Healthcare workers should follow their local regulations to select the best device.
Viral infectionsViruses are frequently causes of occupational infections. Personal protective equipment is considered a pivotal part of safety programs for healthcare personnel at risk of exposure especially when no effective therapies or vaccines are available. 7,16,17
Influenza viruses are among the most commonly studied pathogens. They are RNA viruses and belong to the Orthomyxoviridae family. Their transmission is mostly mediated by droplets through coughing or sneezing, but also by airborne droplet nuclei or by contact, although the importance of the airborne route is controversial. Coronaviruses share some characteristics with Influenza viruses, such as RNA genome. They belong to the Coronaviridae family, known as cause of common cold, but also of other respiratory tract infections, such as severe acute respiratory syndrome (SARS). Their transmission happens through large droplets or by touching contaminated surfaces and fomites, with uncertainties about airborne route.18 A recent study has detected NL63, OC43 and HKU1 coronaviruses RNA in airborne droplets nuclei contained in four participants’ exhaled breaths, out of ten samples. 19
The recent pandemic spread of the novel coronavirus SARS-CoV-2 has pushed the scientific community to a better understanding of the transmission routes. To date, the human-to-human transmission of SARS-CoV-2 is reasonably caused by respiratory large droplets and contact route, but data are insufficient to exclude airborne droplet nuclei transmission. 20
Inward protection: protecting the wearer from the environmentFiltering facepiece respirators provide inward protection, defined as the capacity to reduce the concentration of airborne particles from the environment to the inner side of the device that is in contact with the upper airways of the wearer. Their protection consists of limiting the inhalational transmission of droplets and aerosols, potentially containing pathogens. While FFR are specifically produced for this purpose, medical masks are registered as medical apparel, for general protection of the wearer.10,11 Moreover, respirators are more expensive than medical masks and healthcare facilities do not usually have stockpiles, thus creating more interest about the detailed differences among these devices, especially during epidemics or pandemics.
The most recent indications of World Health Organization (WHO) about the use of PPE during COVID-19 pandemic, recommend the use of respirator N95 or equivalent FFP2 only in the context of aerosol-generating procedures performed on patients with COVID-19, while medical masks are recommended for the general care of patients with COVID-19 provided that they are wearing a medical mask. 21 Aerosol-generating procedures are currently intended for cardiopulmonary resuscitation, tracheal intubation or suction, tracheotomy, manipulation of oxygen masks, bronchoscopy, non-invasive ventilation or, although controversial, use of high flow nasal cannula. 21–23 Worth noting, collecting diagnostic respiratory samples (e.g. nasopharyngeal swab) should be considered as aerosol-generating procedures, because it can cause coughing and/or sneezing. Thus, the healthcare worker in charge for these procedures should wear FFR. 7
In its latest technical report, The European Center of Diseases Control (ECDC) stated that healthcare workers in contact with a suspected or confirmed COVID-19 case should wear a medical mask or, if available an FFP2 respirator. 7
Most of the evidence about the use of PPE in viral infections is based on Influenza models. Despite the known similarities in term of transmission, they should be considered with caution in the context of SARS-CoV-2 infections. A recent systematic review and meta-analysis evaluated the effectiveness of N95 respirators versus medical masks for the prevention of influenza. It included six randomized clinical trials for a total of 9171 participants. No significant differences were found between the use of N95 respirators and medical masks for the outcomes of laboratory confirmed respiratory viral infections (RR=0.89, 95% CI 0.70-1.11), laboratory-confirmed influenza (RR=1.09, 95% CI 0.92-1.28), laboratory-confirmed respiratory infection (RR=0.74, 95% CI 0.42-1.29) or influenza like illness (RR=0.61, 95% CI 0.33-1.14). One of the trials included was actually done in a household setting. The authors concluded suggesting that N95 respirators should not be recommended for the general public and healthcare workers performing low-risk procedures. 24
Specific evidence regarding SARS-CoV-2 is ongoing. A randomized multicentre controlled trial (NCT04296643) in Canada is underway to compare the use of either medical masks or N95 respirator in 576 nurses involved in the care of patients with COVID-19. The primary outcome is laboratory confirmed COVID-19 among the participants.
In addition to FFR, a PPE ensemble usually includes boots, gowns, gloves, hoods and goggles or face-shield. 21 These components seem to provide an additional inward protection, despite the amount of extra protection given by goggles or face-shield remaining controversial, especially in the case of lengthy exposure to airborne droplet nuclei. 25
For household settings, WHO has recommended the use of medical masks, gowns, gloves and eye protection for healthcare workers providing home care to patients with COVID-19, and medical masks for both caregivers and patients. 21,26
Literature exists about the use of masks in household setting, but showing no significant reductions in household transmission, except for some benefit in the case of early application after the onset of the index patient's symptoms. These findings may be partially explained by the lack of compliance with the intervention. 27–29
An interesting pilot study has evaluated N95 respirator and medical masks in the setting of home care.30 The participants were three nurses, involved in healthcare assistance at a patient's home. The workplace protection factor was the primary outcome of the study, defined as Cout/Cin, i.e. the ratio between the aerosol concentrations inside (Cin) and outside (Cout) the device. The measurement of workplace protection factor was repeated twice for each participant, one with N95 respirator and one with medical mask. N95 respirators provided higher respiratory protection in comparison to medical masks, but the protection factors varied on an individual basis, also depending on the activity performed, with a greater risk in specific tasks like tracheal suctioning or nebulizer treatments. 30
Van der Sande et al compared three types of masks (home-made masks, medical masks and FFP2) in their study conducted on healthy volunteers. The performance of the three devices was evaluated, at first, on 28 volunteers, including children, for 10-15minutes. Then, another group of 22 volunteers wore the masks for 3hours during the execution of common activities. Protection factor remained stable over time, not dependent on activity or on changes in the respiratory rate. Although hand-made masks have shown to provide some protection, FFP2 respirators had better protection factors over both medical masks and hand-made masks. 31
Outward protection or source barrier: protecting the environment from the wearerMedical masks are meant to provide an outward protection, thus witnessed by the recent indications about the use of medical masks in COVID-19 patients and symptomatic people visiting a healthcare setting 21,26. Diaz and Smaldone were the first to evaluate the role of medical masks in respiratory source control.32 In a bench study, they found that applying a medical mask on the source could significantly reduce the exposure of the receiver to a radiolabeled aerosol, providing a higher protection than a N95 respirator worn by the receiver. The study was conducted in a chamber designed with 6 air exchanges per hour to permit both dilution and deflection of exhaled particles, and the effect was lost at 0 air exchange per hour. Since the effectiveness of respirators is strongly dependent on their capacity to seal to the face, a more recent bench study has used modern and more fitting mannequins (‘Resusci Anne’ – Laerdal) and confirmed the role of medical masks for source control.33 The role of medical masks for the source control of SARS-CoV-2 infected patients is controversial. A recent study showed a lower seasonal coronavirus RNA detection rate in droplets and aerosol samples collected from participants wearing a medical mask, compared to participants not wearing a mask.19 In contrast with these findings, a study has been conducted on four patients with COVID-19 invited to cough onto a petri dish. Each participant repeated the experiment with no mask, a medical mask and a cotton-mask. Differences in SARS-CoV-2 viral loads on the petri dishes were not significant across the combinations and the virus was detected on the external surfaces of the masks, thus demonstrating a potentially insufficient outward protection. 34
Lack of supplies and re-useThe tragic conditions of a pandemic can increase the demand for PPE, potentially exceeding its availability. On 19th of March 2020, WHO confirmed that “The current global stockpile of PPE is insufficient, particularly for medical masks and respirators; the supply of gowns and goggles is soon expected to be insufficient also”, as already stated also in the precedent versions of its document ‘Interim guidance on COVID-19’. 21
WHO has recommended an extended use of PPE, i.e wearing the same respirator for repeated close contacts in the case of cohorted patients with COVID-19. 21 However, no recommendations were released about the re-use or decontamination of disposable PPE. The hypothesis of the re-use has been evaluated by several bench studies over time and interesting data about residual efficacy and performance post decontamination of disposable respirators are available.
The U.S. Center for Disease Control and Prevention has provided a summary of the evidence available on decontamination procedure of disposable FFR. It is clearly stated that the use of a decontaminated FFR should be a part of a crisis capacity strategy. Vaporous hydrogen peroxide, ultraviolet germicidal irradiation, and moist heat are listed among the methods that may preserve filtering performance. A recommendation is made against the use of autoclave, 160°C dry heat, 70% isopropyl alcohol, microwave irradiation, bleach and soap and water, because they could affect the filtering performance. 35
In 2012, Lore et al. examined the effectiveness of three energetic decontamination methods (ultraviolet germicidal irradiation, microwave-generated steam, and moist heat) on two models of N95 respirators, previously contaminated with H5N1.36 Post decontamination viral load results decreased with all the methods. Filter performance was also tested, and no alterations were registered. These findings were in line with data on six respirators with H1N1 contamination.37 Other studies have observed physical degradation of respirator materials such as the fibers composing the body material, which is probably less resistant than the filters.38 Thus, the decontamination should balance the need for inactivation of the specific pathogen, even from the interior layers of the respirator, and the need to preserve the filtering performance, the structure of the respirator and its fitting characteristics. Further studies are needed to clarify the safety profiles of re-using procedures of disposable PPE. Meanwhile, if re-use is needed, the user should check that the decontamination tests have already been performed on the specific model of FFR.
The use of cloth-masks in comparison with medical mask and usual protection, has also been investigated by a multicentre cluster randomized trial, including 1607 healthcare workers. The trialshowed a significantly higher rate of ILI in the cloth-mask intervention group (RR=13.00, 95% CI 1.69 to 100.07) compared with the medical mask intervention group and with the entire control group, where healthcare workers wore their usual protection (including N95 respirator, no mask, medical mask, cloth mask or a combination of medical and cloth mask). 39
Performance of healthcare workers using PPEAlthough necessary, the use of PPE can also influence the performance of healthcare workers, as observed in studies on cadaveric models, where a higher difficulty rate and a lower success rate of tracheal intubations were registered.40 In contrast with these findings, a clinical study conducted during SARS outbreak, found no significant differences in the overall tracheal intubation-related complication rate comparing the periods pre-SARS, during SARS and post-SARS. 41
With the aim saving on supplies, extended use of PPE has been recommended, but healthcare personnel may experience intolerance to wearing a respirator for the entire work shift, even with interposed break periods.42 Wearing a medical mask over the FFR has also been proposed, to enhance the duration of FFR, but concerns exist regarding negative effects on the wearer. 43
Filtering facepiece respirators are used for several uninterrupted hours (about 8hours) in non-healthcare settings. The maximum length of use in healthcare settings is typically dictated by other factors, such as contamination occurring, wearer intolerance or need to use the rest period, rather than by a predetermined duration. 44
The need to reduce the exposure time and to use the smallest possible number of PPE reinforces the restriction on the number of healthcare personnel caring for the infected patients. This is of outmost important during aerosol-generating procedures, especially airway management, which should be performed by the most skilled available operators.45 The downside of this approach is the reduced exposure of junior doctors to these procedures. This phenomenon has been already observed during SARS outbreak, and continued in the immediate post-outbreak periods, with concerns about training quality and preparedness of young personnel in the management of future similar situations. 41
Finally, although equipped with protective clothing and even being experienced for the required tasks, healthcare workers can also be contaminated by improper removal of PPE,46 thus regular training should be implemented to guarantee the best protection. Filtering facepiece respirators should be removed after the removal of the other components of PPE, just before removing of the gloves. The anterior part should not be touched during removal.47 It is important to note doffing is done at the end of a shift, when an already fatigued worker can easily make mistakes during the procedure. A lot of human and organizational risk factors can co-exist, and appropriate knowledge of the procedure, plus the presence of a doffing partner are suggested.
ConclusionsIn general, clinical evidence on the use of FFR is poor. The use of appropriate PPE, such as FFR, is of pivotal importance for the healthcare workers involved in the care of patients with viral infections, such as the currently pandemic, COVID-19. Unfortunately, supplies are rapidly running out worldwide. Moreover, knowledge about specific characteristics of FFR is of utmost importance to select the proper type according to the clinical setting. Direct evidence on the effectiveness of FFR in the prevention of SARS-CoV-2 infection is low and still underway, with concerns about the generalizability of other virus models. Available literature seems to reflect the alternating of periods of peak interest, coinciding with epidemics, with periods of lack of interest towards the topic. There should be a more constant research effort which also addresses the need for periodical training of healthcare workers.
Authors’ contributionMI conceived the content, drafted the manuscript, approved the final version to be submitted. FV, CG, GA, PI, AG helped in writing the manuscript and revised it critically for important intellectual content, approved the final version to be submitted. AC conceived the content, drafted the manuscript, approved the final version to be submitted.
Competing interestsAll authors declare no competing interests.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
None