An increasing body of evidence from clinical trials and real-world studies suggests that metronomic oral vinorelbine (VNR) is a promising treatment option for elderly and unfit advanced non-small cell lung cancer (NSCLC) patients. The aim of this multicenter study was to present real-world data about the experience in treatment of NSCLC with metronomic VNR in Portugal.
Material and methodsRetrospective data from NSCLC patients not eligible for conventional chemotherapy or tyrosine kinase inhibitors who received oral metronomic VNR irrespective of treatment line and dose was retrieved from 19 Portuguese Oncology Centers between 2016 and 2018.
ResultsA total of 293 patients were included, with a median of 76 (39 − 94) years; 71% were ≥70 years old. Patients had a median of 3 comorbidities and predominantly (61%) ECOG PS 2. Most (42%) received metronomic oral VNR as first-line treatment. Overall response rate was 18%, with 42 (18%) partial and no (0%) complete responses. A total of 54% of patients experienced stable disease and 28% of patients, disease progression. Disease control rate was 72%. Patients were a median of 4 (1 − 40) months on treatment. Treatment discontinuation was observed in 90%, mostly (67%) due to disease progression, followed by death (16%). Adverse events leading to treatment discontinuation were only reported in 5% of patients. Female gender (HR 0.601, 95% CI 0.434 − 0.832; p = 0.002) and ECOG PS 1 (HR 0.625, 95% CI [0.443 − 0.881]; p = 0.007) were significantly associated with a lower risk of metronomic oral VNR discontinuation. Overall, 21% of patients experienced G3/4 toxicity.
ConclusionThe present real-world results agree with what has been previously reported by other international Centers and support the concept that metronomic scheduling is a relevant and safe approach to treat advanced NSCLC patients.
Lung cancer remains one of the most commonly diagnosed cancer and a leading cause of mortality worldwide.1 According to Globocan, the malignancy accounted for 11.6% of total cancer cases and 18.4% of total cancer deaths in 2018, representing a substantial health burden.1 Non-small cell lung cancer (NSCLC), the dominant subtype, accounts for 80 − 90% of cases,2,3 and a remarkable proportion of these patients (≈70%) presents with metastatic or locally advanced disease.4,5
Approximately half of lung cancers are diagnosed in people aged ≥70 years, and around 15% of cases in people aged ≥80 years.6 With the aging of populations, this trend will predictably increase in upcoming years. Age is a relevant independent prognostic factor affecting patient survival.6,7 Furthermore, the elderly are frequently frail, with poor performance status and multiple comorbidities, and often ineligible for conventional cytotoxic chemotherapy, associated with important toxicities.8–11 For these reasons, treatment of elderly patients with advanced NSCLC can be challenging.
For advanced NSCLC patients without actionable oncogenic drivers (EGFR-sensitizing mutations, ALK rearrangements, ROS1 translocation, or BRAF V600 mutation), the updated 2019 guidelines of the European Society for Medical Oncology (ESMO) recommend platinum (preferably carboplatin)-based doublet chemotherapy for eligible elderly patients and for those with Eastern Cooperative Oncology Group [ECOG] performance status (PS) 0–2 and adequate organ function, and single-agent chemotherapy with gemcitabine, vinorelbine, docetaxel, or pemetrexed (in non-squamous NSCLC) for patients not eligible for doublet chemotherapy.2,3 Regarding immune checkpoint inhibitors, ESMO guidelines consider that there is insufficient data but permit their use in ECOG PS 2 patients, but also advise on their use according to standard recommendations in elderly patients.2,3
Metronomic chemotherapy, the frequent or continuous administration of low-dose chemotherapy with no or short drug-free intervals between single administrations, allows for a prolonged treatment with potentially less toxicity.12,13 The rationale behind metronomic administration is to improve the therapeutic index by balancing drug activity and treatment-associated toxicities, prolonging treatment duration and improving quality of life (QoL). This schedule was designed to overcome acquired tumour resistance to chemotherapy and has multiple mechanisms of action,14 including antiangiogenic, cytostatic, and immunomodulating effects,15–17 which allow the delay of cancer progression while reducing toxicity and decreasing the need for growth factor agents to recover from myelosuppression.15 This profile makes this agent particularly suitable for elderly and/or fragile patients.
Vinorelbine (VNR), a semisynthetic vinca-alkaloid, was originally formulated as an intravenous (i.v.) agent, but later also as an oral treatment option. It was the first agent studied in mono-chemotherapy in elderly NSCLC patients: in the ELVIS study, the classical i.v. VNR schedule plus best supportive care (BSC) showed a survival benefit compared with BSC alone (median of 28 vs 21 weeks; HR 0.65; 95% confidence interval [CI] 0.45–0.93),18 while in the study by Gridelli et al., oral VNR demonstrated good clinical outcomes with the classical administration in these patients.19 Oral VNR has already shown significant activity in different NSCLC settings, either in combination or monotherapy, including concurrent chemoradiation for locally advanced disease, adjuvant treatment for resected disease, and palliative chemotherapy for recurrent/metastatic disease.20
Metronomic oral VNR has also been investigated among elderly NSCLC patients, both in clinical trial and real-life setting. The optimal dose for metronomic VNR administration was established as 50 mg given three times a week.21,22 Metronomic oral VNR has demonstrated interesting activity and safety in elderly patients with advanced NSCLC in phase I/II trials.22–26 In real-life setting, emerging retrospective data confirms the effectiveness and absence of relevant safety issues of metronomic oral VNR, and its applicability for patients unfit for standard chemotherapies.27–32 More recently, metronomic oral VNR has also been evaluated as a switch maintenance regimen versus BSC in patients with advanced NSCLC who did not progress after first-line platinum-based chemotherapy in the phase II randomized MA.NI.LA study.33 In this setting, the metronomic VNR schedule prolonged progression-free survival (PFS) compared to BSC, particularly in patients aged ≥70 years and in those with disease stabilization after induction chemotherapy. However, the dropout rate due to significant toxicity raises awareness of the need to further investigate the optimal metronomic oral VNR dose after induction chemotherapy.
According to the Spanish Working Group on Geriatric Oncology of the Spanish Society of Medical Oncology (SEOM), metronomic oral VNR is a suitable option for the treatment of elderly NSCLC patients.34 And, overall, evidence is building on the benefit of metronomic oral VNR in the relevant proportion of frail/unfit NSCLC patients often excluded from clinical trials.
The aim of this multicenter study was to present real-world, retrospectively collected data about the experience in treatment of NSCLC with metronomic VNR in Portugal. Effectiveness (overall response rate [ORR] and treatment duration) and safety data from Portuguese NSCLC patients treated with metronomic oral VNR is presented.
Material and methodsThis study was an observational, retrospective, real-world analysis of metronomic oral VNR in the treatment of advanced NSCLC patients. Data included NSCLC patients not eligible for conventional chemotherapy who received oral metronomic VNR irrespective of treatment line and dose, given three times a week for ≥1 complete treatment cycle. Information about disease progression, patient refusal, unacceptable toxicity, or death was collected from patients’ clinical records in 19 Portuguese cancer-treating institutions in the mainland and islands from when VNR started to be routinely used in national clinical practice (2016) until December 2018. No formal ethical approval was required, given the study’s retrospective and observational nature. All patient data was coded and secure.
Retrieved data included patients’ (i) baseline clinical and demographic features (age, gender, tumour histological type), (ii) clinical characteristics at metronomic oral VNR start (number of comorbidities, Eastern Cooperative Oncology Group [ECOG] performance status [PS], prior treatment regimens), and (iii) metronomic oral VNR treatment data (VNR dose, best response according to RECIST criteria,35,36 overall survival [OS], treatment duration, adverse events, and treatment discontinuations).
Outcome measures considered in the analysis included treatment duration, overall response rate (ORR), disease control rate (DCR), and toxicity. ORR was defined as the proportion of patients achieving complete or partial response as best overall response according to RECIST 1.1 criteria. DCR was defined as the percentage of patients presenting complete, partial, or stable disease.
Treatment was discontinued after disease progression or unacceptable toxicity, death, or patient preference. Treatment duration was defined as time since treatment start and discontinuation.
Some patients started treatment with the 50 mg dose and others with lower doses, which could be changed. Information of the lower delivered dose was also collected.
Adverse events were recorded according to the Common Terminology Criteria for Adverse Events (CTCAE) v4.0.37 Patients could receive palliative treatment as required according to local clinical practice but were excluded if concomitantly receiving other anticancer agents.
Statistical analysisCategorical variables were expressed as absolute and relative frequencies, and continuous variables as median and interquartile range (IQR).
Kaplan-Meier curve was estimated for treatment duration, and proportional hazard (or Cox) models were fitted to data to investigate whether patients’ baseline demographic or clinical characteristics were associated with a higher risk of treatment discontinuation or death. The assumption of proportional hazards was tested, and presence of time-dependent covariates was assessed using Aalen models. The level of significance was set at p < 0.05.
ResultsA total of 293 patients, mostly male (75%), were enrolled in this study, with a median of 76 (range 39 − 94) years. Overall, 29% of patients were <70 and 71% were ≥70 years of age.
Baseline (i.e. at time of VNR start) characteristics of the study cohort are depicted in Table 1. Most patients (94%) had ≥1 comorbidities, mostly (45%) between 2 and 3. The median number of comorbidities was 3 (range 0 − 9). Patients with ECOG PS 2 were predominant (61%), with an also relevant proportion of patients with ECOG PS 3 (17%). Around 20% of patients with ECOG PS 1 were included. These were patients with important comorbidities contraindicating the use of other therapies or who had received prior treatments. Non-squamous histology was prevalent (66%), followed by squamous histology (30%), and few patients (4%) presented with not otherwise specified (NOS) carcinoma.
Baseline characteristics of the study population.
| Characteristic | N = 293 |
|---|---|
| Age − years | |
| Median (range) | 76 (39 − 94) |
| Gender − n (%) | |
| Male | 221 (75) |
| Female | 72 (25) |
| Comorbidities − n (%) | |
| 0 | 18 (6) |
| 1 | 49 (17) |
| 2 | 70 (24) |
| 3 | 61 (21) |
| 4 | 47 (16) |
| 5 | 27 (9) |
| 6 | 14 (5) |
| 7 | 6 (2) |
| 8 | 0 (0) |
| 9 | 1 (0) |
| ECOG PS − n (%) | |
| 0 | 5 (2) |
| 1 | 60 (20) |
| 2 | 179 (61) |
| 3 | 49 (17) |
| Tumour histology − n (%) | |
| Squamous | 88 (30) |
| Non-squamous | 193 (66) |
| NOS | 12 (4) |
ECOG PS, Eastern Cooperative Oncology Group performance status; NOS, not otherwise specified.
Most patients (42%) received metronomic oral VNR as first-line treatment, followed by 33% who received it as second line, 15% as third line, and a minority as fourth and subsequent lines of treatment (Table 2). Metronomic oral VNR initial treatment schedule was 40 mg per administration in 67% of patients, 50 mg per administration in 24% of patients, and 30 mg per administration in 9% of patients. Maximum delivered dose was 40 mg in 61% of patients and 50 mg in 33% of patients. Minimum delivered dose is also presented, as some patients required initial dose reductions due to intolerance or toxicity. The most used schedule for first-, second-, and subsequent-line treatment was 40 mg per administration (27%, 24%, and 17%, respectively).
Metronomic oral VNR effectiveness and safety data.
| Characteristic | N = 293 |
|---|---|
| Previous treatment regimens − n (%) | |
| 0 | 123 (42) |
| 1 | 97 (33) |
| 2 | 45 (15) |
| 3 | 17 (6) |
| 4 | 6 (2) |
| 5 | 2 (1) |
| 6 | 3 (1) |
| VNR initial dose (mg/adm) − n (%) | |
| 30 | 26 (9) |
| 40 | 197 (67) |
| 50 | 70 (24) |
| VNR lowest dose (mg/adm) − n (%) | |
| 30 | 37 (13) |
| 40 | 199 (68) |
| 50 | 57 (19) |
| Best response − n (%) | |
| CR | 0 (0) |
| PR | 42 (18) |
| SD | 126 (54) |
| PD | 66 (28) |
| Treatment duration − months | |
| Median (range) | 4 (1 − 40) |
| Treatment discontinuation − n (%) | 264 (90) |
| Reasons for discontinuation − n (%)a | |
| Disease progression | 178 (68) |
| Adverse events | 14 (5) |
| Death | 43 (16) |
| Patient preference | 10 (4) |
| Others | 19 (7) |
| Adverse events − n (%) | Grade 1/2 | Grade 3/4 |
|---|---|---|
| Any event | 62 (21) | |
| Hematological events | ||
| Anemia | 90 (30.7) | 13 (4.4) |
| Neutropenia | 31 (10.6) | 30 (10.2) |
| Febrile neutropenia | 2 (0.7) | 15 (5.1) |
| Non-hematological events | ||
| Diarrhea | 36 (12.3) | 7 (2.4) |
| Fatigue | 91 (31.1) | 28 (9.6) |
| Nausea/vomiting | 44 (15.0) | 5 (1.7) |
VNR, vinorelbine; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Overall response rate (ORR) with metronomic oral VNR was 18%, with 42 (18%) partial responses (PR) and no complete responses (CR). A total of 54% of patients experienced stable disease (SD) and 28% of patients experienced disease progression (PD). Disease control rate (DCR) was 72%. Patients were a median of 4 (range 1 − 40) months on treatment with metronomic oral VNR. Treatment discontinuation was observed in 90%, mostly (67%) due to disease progression, followed by death (16%). Adverse events leading to treatment discontinuation were observed in only 5% of patients.
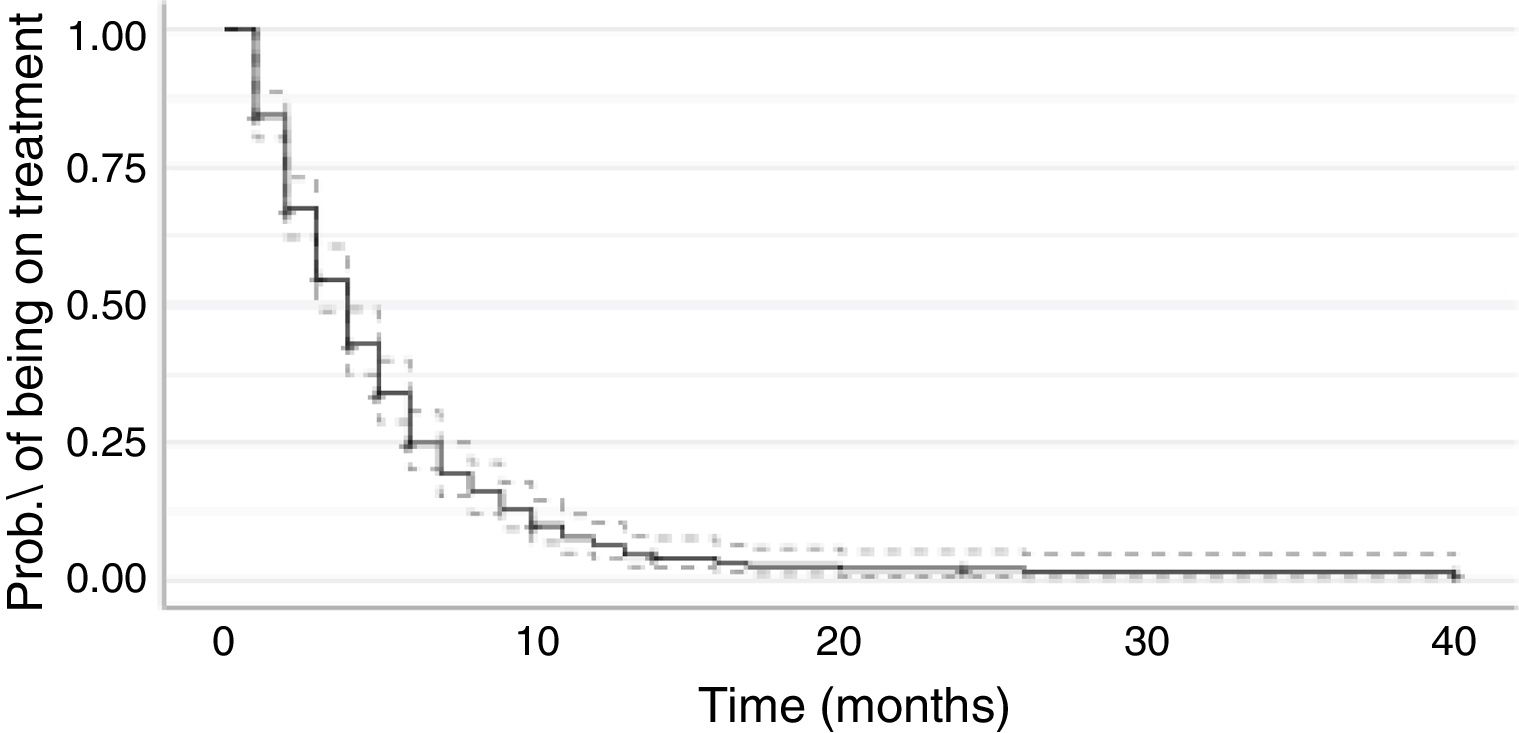
The Kaplan-Meier curve for metronomic oral VNR treatment duration is shown in Fig. 1.
Females (median 5 months, 95% CI 4 − 6), patients with no comorbidities (median 5 months, 95% CI 3 − 10), with ECOG PS 1 (median 5 months, 95% CI 3 − 7), and with a VNR starting dose of 50 mg/administration (median 5 months, 95% CI 4 − 6 months) were a longer time on treatment with metronomic oral VNR (Table 3). Median treatment duration for ECOG PS 3 patients was 4 months (95% CI 3 − 6).
Median time on treatment with metronomic oral VNR.
| Characteristic | N = 293 | Events | Time on VNR treatment | |
|---|---|---|---|---|
| Median | 95% CI | |||
| Global | 293 | 264 | 4 | 3 − 4 |
| Gender | ||||
| Male | 221 | 199 | 4 | 3 − 4 |
| Female | 72 | 65 | 5 | 4 − 6 |
| Nr. comorbidities | ||||
| 0 | 18 | 15 | 5 | 3 − 10 |
| 1 | 49 | 45 | 4 | 3 − 5 |
| 2 | 70 | 64 | 4 | 3 − 5 |
| 3 | 61 | 58 | 4 | 3 − 6 |
| 4 | 47 | 41 | 4 | 2 − 5 |
| 5 | 27 | 24 | 3 | 2 − 5 |
| 6 | 14 | 12 | 3 | 1−NE |
| 7 | 6 | 4 | 5 | 4−NE |
| 8 | 1 | 1 | 5 | NE − NE |
| 9 | ||||
| ECOG PS at VNR start | ||||
| 0 | 5 | 5 | 3 | 1−NE |
| 1 | 60 | 46 | 5 | 3 − 7 |
| 2 | 179 | 165 | 4 | 3 − 4 |
| 3 | 49 | 48 | 4 | 3 − 6 |
| Histology | ||||
| Squamous | 88 | 78 | 4 | 3 − 5 |
| Non-squamous | 193 | 175 | 4 | 4 − 5 |
| NOS | 12 | 11 | 2 | 1−NE |
| Previous regimens | ||||
| 0 | 123 | 108 | 4 | 3 − 5 |
| 1 | 98 | 93 | 4 | 4 − 5 |
| 2 | 45 | 42 | 4 | 3 − 6 |
| >3 | 28 | 21 | 3 | 2−NE |
| VNR initial dose (mg/adm) | ||||
| 30 | 26 | 22 | 3 | 2 − 4 |
| 40 | 197 | 182 | 4 | 3 − 4 |
| 50 | 70 | 60 | 5 | 4 − 6 |
| VNR minimum delivered dose (mg/adm) | ||||
| 30 | 37 | 32 | 3 | 3 − 4 |
| 40 | 199 | 184 | 4 | 3 − 5 |
| 50 | 57 | 48 | 4 | 4 − 6 |
VNR, vinorelbine; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; NOS, not otherwise specified; NE, not estimated.
Features significantly associated with a lower risk of metronomic oral VNR treatment discontinuation were female gender (HR 0.601, 95% CI 0.434 − 0.832; p = 0.002) and ECOG PS 1 (HR 0.625, 95% CI [0.443 − 0.881]; p = 0.007; Table 4). Additionally, the number of comorbidities and prior lines of treatment were not associated with a change in metronomic oral VNR treatment time.
Proportional hazard coefficients for metronomic oral VNR treatment discontinuation.
| Characteristic | eβi | p | |
|---|---|---|---|
| Estimate | 95% CI | ||
| Age | 0.991 | 0.974 − 1.007 | 0.272 |
| Gendera | |||
| Female | 0.601 | 0.434 − 0.832 | 0.002 |
| Nr. comorbidities | 0.977 | 0.905 − 1.056 | 0.560 |
| ECOG PSb | |||
| 0 | 1.973 | 0.773 − 5.033 | 0.155 |
| 1 | 0.625 | 0.443 − 0.881 | 0.007 |
| 3 | 0.852 | 0.594 − 1.222 | 0.384 |
| Histologyc | |||
| Non-squamous | 0.745 | 0.549 − 1.010 | 0.058 |
| NOS | 2.028 | 1.044 − 3.937 | 0.037 |
| Prior lines of treatmentd | |||
| 1 | 0.843 | 0.611 − 1.163 | 0.298 |
| 2 | 0.971 | 0.639 − 1.474 | 0.889 |
| >3 | 1.308 | 0.766 − 2.233 | 0.325 |
| VNR initial dose (mg/adm)e | |||
| 30 | 1.315 | 0.511 − 3.384 | 0.570 |
| 50 | 0.893 | 0.535 − 1.489 | 0.664 |
| VNR minimum delivered dose (mg/adm) | |||
| 30 | 1.064 | 0.544 − 2.083 | 0.855 |
| 50 | 0.971 | 0.578 − 1.630 | 0.910 |
Concerning toxicities, among registered events most patients (46%) experienced grade 1/2 toxicity, including 31.1% of G1/2 fatigue, 30.7% of G1/2 anaemia, and 15.0% of G1/2 nausea or vomiting. A total of 21% of patients reported grade 3/4 toxicity, mostly G3/4 neutropenia (10.2%) and G3 fatigue (9.6%; Table 2), and 33% of patients experienced no toxicity with metronomic oral VNR.
Sixty-three patients required granulocyte colony-stimulating factors (GCSF) for neutropenia, accounting for 41% of all patients who developed neutropenia of any grade and for 9% of the overall study population.
DiscussionThe present study evaluated treatment outcomes of 293 advanced/metastatic NSCLC patients undergoing a metronomic oral VNR regimen, three times a week until progression, patient refusal, unacceptable toxicity, or death according to the approved indication.
The oral VNR formulation appears to have a similar effectiveness and better safety profile compared with its intravenous counterpart and enabled the use of a metronomic schedule, which has the advantages of being cost-sparing in terms of hospital expenditures, easier to prepare and administer (namely considering infusion-related procedures), and represents a more convenient approach for patients and for patient management. The oral thrice weekly delivery results in a comparable delivery to weekly parenteral dosing, although leading to a more protracted exposure to lower drug concentrations.26
The NSCLC cohort included in this study had a high median age (76 years), several comorbidities (median of 3), and a high percentage of PS 2 patients (61%). Additionally, most patients (42%) received metronomic oral VNR as first-line treatment. Overall, this data is consistent with the profile of patients with advanced NSCLC unfit for polychemotherapy but still eligible for active treatment.
About 20% of patients with ECOG PS 1 were included. These were patients with important comorbidities or who had received prior treatments that contraindicated the use of other therapies.
Patients without comorbidities, good ECOG PS, and a VNR starting dose of 50 mg/administration reported longer time on treatment with the metronomic VNR schedule. The optimal monotherapy VNR dose can vary between 30 and 50 mg. Due to pre-existing comorbidities, the 40 mg dose was started in most patients and maintained, as patients displayed good tolerability and no adverse events. Indeed, most patients required no dose reductions.
Taking into account the specific characteristics of this population, it is noteworthy that most patients had their disease stabilized for a relevant period of time, with a DCR of 72% for the entire cohort.
Results here reported are consistent with those previously described by other authors. In the phase II MOVE trial, investigating metronomic oral VNR at the dose of 50 mg as first-line treatment for elderly patients with advanced NSCLC, median age was 80 years, 62.8% of patients had ECOG PS 2, and median time to disease progression was 5 months.22
Also similarly to the present study, the most frequent all-grade non-hematological toxicity in that trial was fatigue (32.4%), with 0.1% of G3/4 fatigue.
In a phase II study of the Hellenic Oncology Research Group, metronomic oral VNR at the dose of 50 mg was administered to advanced NSCLC patients in second line and beyond.25 Patients’ median age (65 years) was lower than in the present study (76 years) and most patients (76.1%) had ECOG PS 1 (versus only 20% in this study). Despite the clinical trial setting, safety outcomes reported in the Hellenic Group study were similar to those reported in the present real-world study, with fatigue and neutropenia being the most frequent non-hematological and hematological G3/4 toxicities reported. In the Hellenic Group study, median time to tumour progression (TTP) was 2.2 months, but the study only included patients treated in second and subsequent lines, while the present study included 42% of patients treated upfront. In a recent retrospective study by Camerini et al., 270 patients with advanced NSCLC were enrolled, with a similar median age to this study’s cohort (76 years).28 Forty-nine percent of patients had PS ≥ 2, compared with 78% of patients in the present study, and 67% were treated in first line versus 42% in this study. Median overall TTP was 5 (range 1 − 21) months, in accordance with the present study. In the study by Camerini, G3/4 toxicity mainly consisted in G3 fatigue and anemia and occurred in 2% of patients.
In this cohort retrieved from daily clinical practice of NSCLC treatment, the main cause of treatment discontinuation was disease progression (67%), with only 5% of patients discontinuing treatment due to adverse events. Additionally, more than 30% of patients experienced no treatment-related toxicities and most reported adverse events were G1/2.
Toxicity is a relevant issue in the palliative setting of NSCLC treatment, and efforts should be made to achieve an optimal balance between the desired treatment benefit (quality of life improvement and/or survival extension) and drug-related toxicity. As here evidenced, the toxicity of metronomic oral VNR was generally well tolerated and easily manageable. Results obtained are clinically relevant, as toxicity-related discontinuation rates were low, and suggest that single-agent metronomic oral VNR has an acceptable clinical effectiveness and safety when used in first and subsequent lines of treatment of advanced NSCLC patients.
As study limitations, it should be acknowledged that this retrospective analysis did not include a control group and that patients enrolled had different numbers of previous lines of treatment. No information was retrieved regarding tumour molecular characteristics, PD-L1 expression, stage, or previous lines of therapy. Due to the number of observed deaths and short follow-up, it was not possible to estimate median OS. Time to progression and PFS data were not collected.
Regarding comorbidities, only the number was registered and no Charlson index or similar was applied. Smoking status was not presented and there is no data in the literature on pharmacokinetics or pharmacodynamics, response, or toxicity changes by smoking status.
Intrinsic to the study’s retrospective nature, the risk of registration bias should also be considered, especially regarding adverse events.
Lastly, study design precluded subgroup analyses by treatment line or performance status, which were not performed.
ConclusionsThe present study reports the experience of 19 Portuguese Centers with the use of metronomic oral VNR in advanced NSCLC patients. Overall, results agree with what has been previously reported by other international Centers and add support to the concept that metronomic scheduling is a relevant and safe approach to treat these patients. Furthermore, it seems to be a legitimate option for ECOG PS 2 and 3 patients with various comorbidities, enabling disease stability for a considerable period of time with good tolerability, low toxicity, and a favorable disease control. Importantly, the main cause of treatment discontinuation with this regimen was disease progression, with only a few patients discontinuing treatment due to safety issues. The number of observed deaths and short follow-up time precluded estimations of median OS or PFS in this study.
The Portuguese experience with metronomic oral VNR here reported, together with the experience of other international Centers, is important to build up evidence on this promising new treatment option.
Future real-world prospective studies are desirable, focusing on the evaluation of predictive factors, use of geriatric assessment tools, and assessment of quality of life and cost-effectiveness associated with this treatment. Also, it would be relevant to investigate the subgroup of patients receiving second-line immunotherapy following first-line metronomic oral VNR, as several studies report a synergistic effect between the two therapies.15,38
FundingThis study was supported by Pierre Fabre Médicament.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors gratefully acknowledge the contribution of the Portuguese clinicians who included patients in this study: Encarnação Teixeira, Paula Alves, Ana Sofia Vilariça (Centro Hospitalar e Universitário Lisboa Norte); Ricardo da Luz, Anuraj Quiran (Centro Hospitalar Lisboa Central); Carlos Lousada (Centro Hospitalar Médio Tejo; Patrícia Garrido (Fundação Champalimaud); Fernando Nogueira (Hospital de Egas Moniz − Centro Hospitalar Lisboa Ocidental); Teresa Cardoso (Hospital do Espírito Santo de Évora); Amélia Brilhante (Hospital Prof. Doutor Fernando Fonseca); José Miguel Lopes (Hospital Garcia de Orta); João Rato (Hospital da Luz Setúbal); Teresa Almodôvar (Instituto Português de Oncologia de Lisboa Francisco Gentil); Carolina Camacho (Hospital Central do Funchal); Alice Pêgo, Ana Figueiredo (Centro Hospitalar e Universitário de Coimbra); Salete Valente (Centro Hospitalar Cova da Beira); Luís Ferreira, Adelino Amaral (Hospital Sousa Martins, Unidade Local de Saúde da Guarda); Joana Godinho (Centro Hospitalar de Entre o Douro e Vouga); Henrique Queiroga (Centro Hospitalar Universitário de S. João); Marta Soares, Sarah Lopes (Instituto Português de Oncologia do Porto Francisco Gentil); José Albino (Unidade Local Saúde Alto Minho).
The authors further acknowledge the medical writing and editorial assistance in the preparation of this manuscript provided by Doctor Joana Cavaco-Silva (jo.cvsilva@gmail.com).