Mid-regional proadrenomedullin (MR-proADM) is a novel biomarker with potential prognostic utility in patients with community-acquired pneumonia (CAP).
PurposeTo evaluate the value of MR-proADM levels at ICU admission for further severity stratification and outcome prediction, and its kinetics as an early predictor of response in severe CAP (SCAP).
Materials and methodsProspective, single-center, cohort study of 19 SCAP patients admitted to the ICU within 12h after the first antibiotic dose.
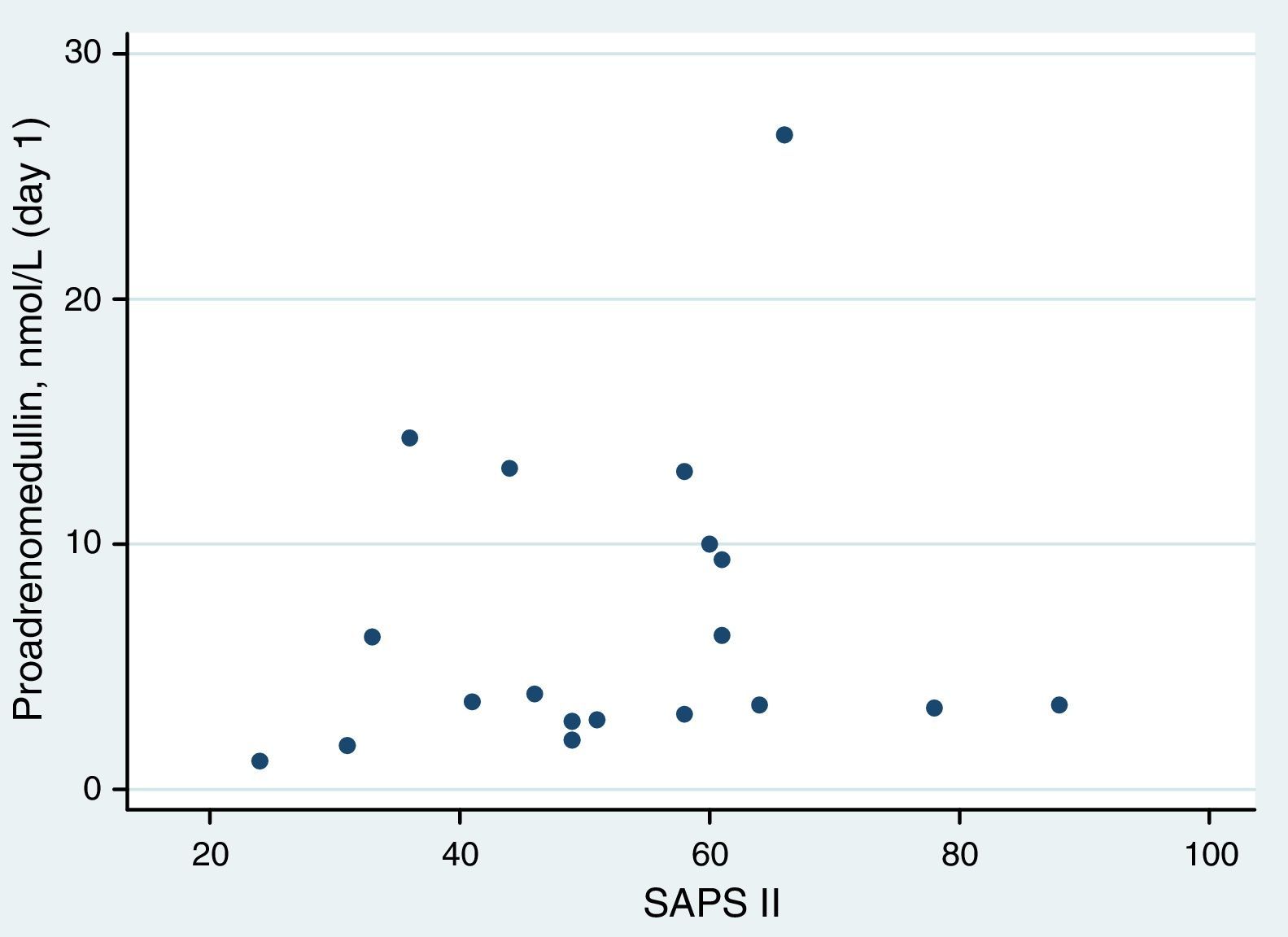
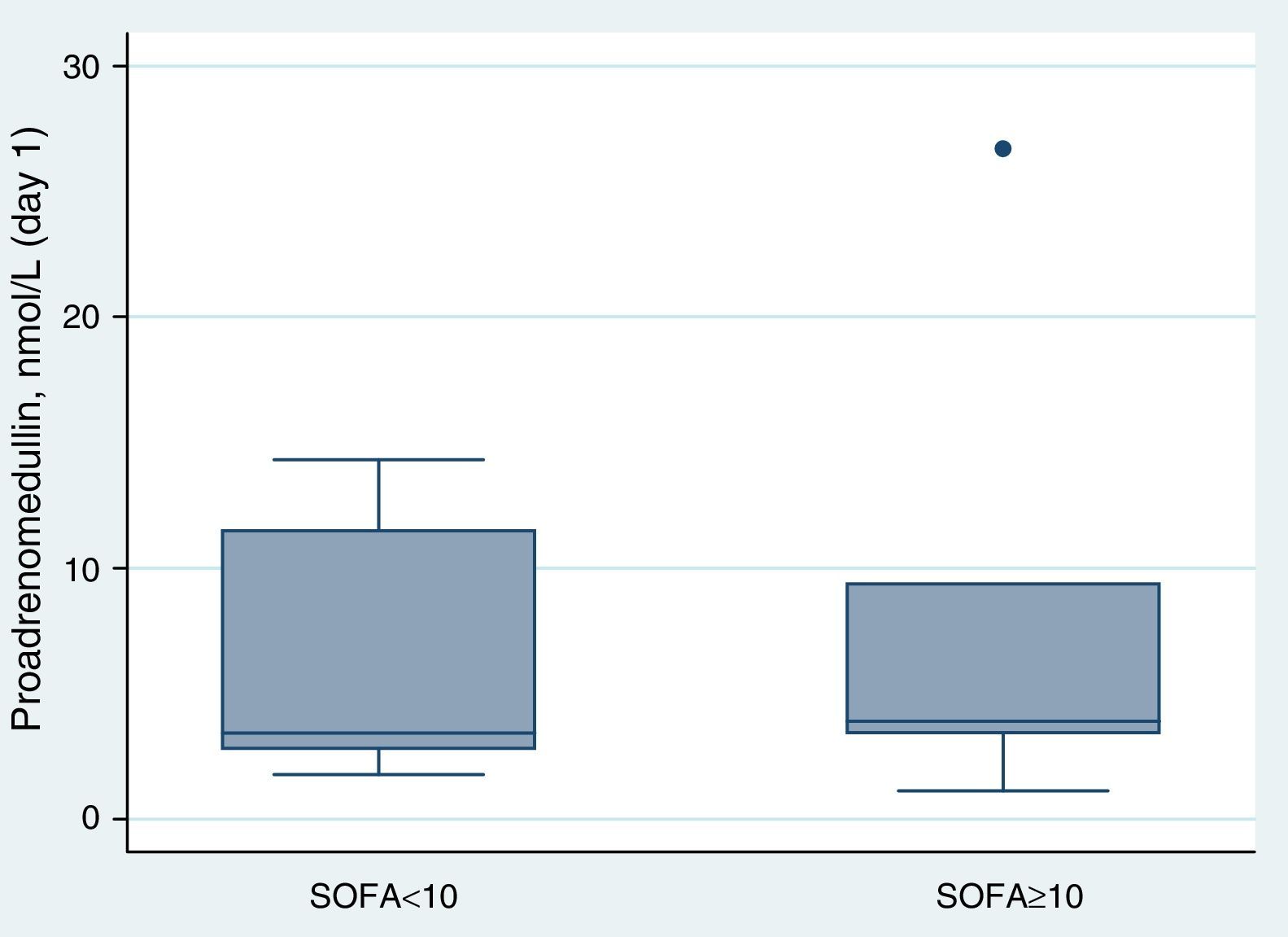
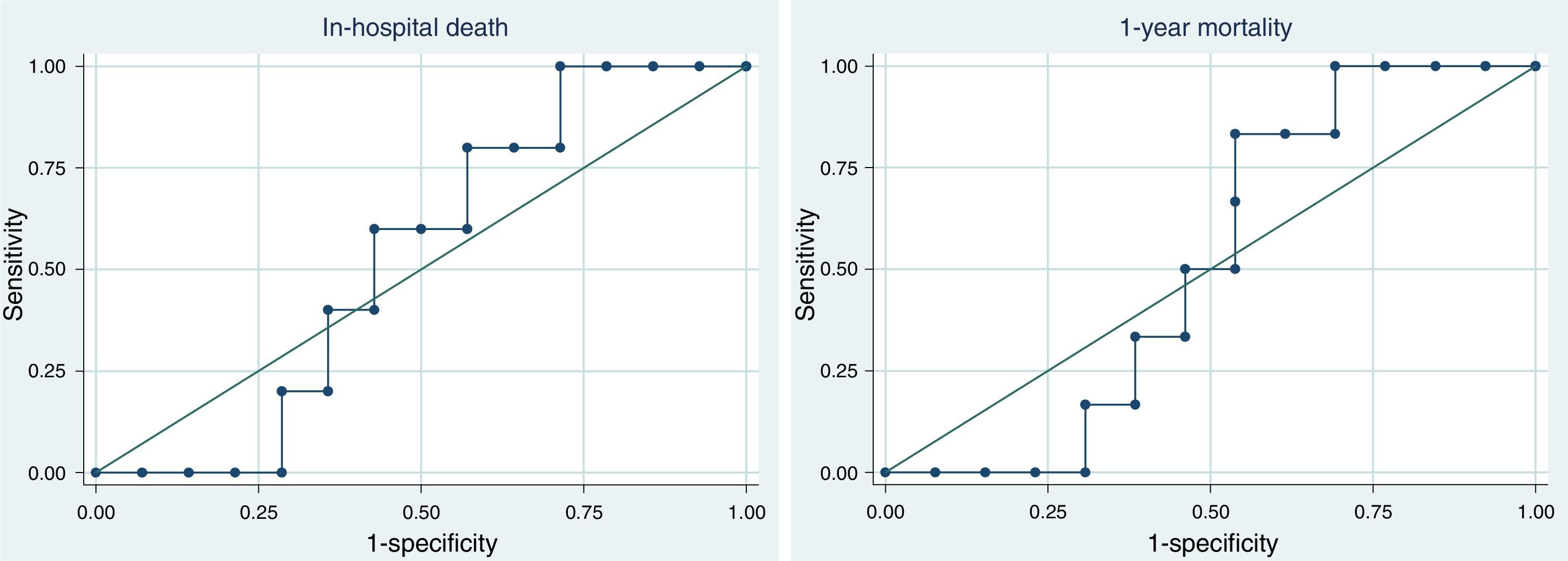
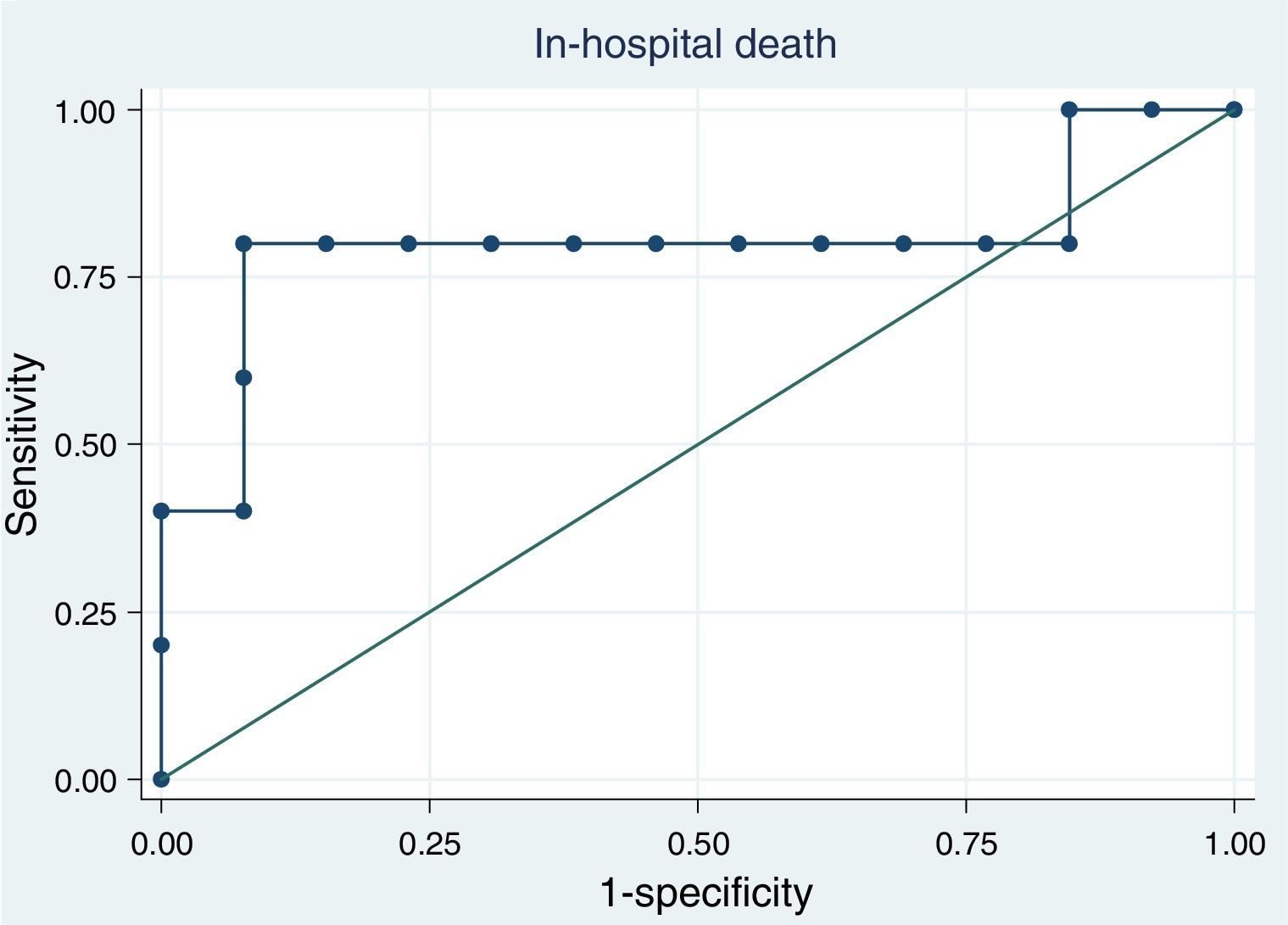
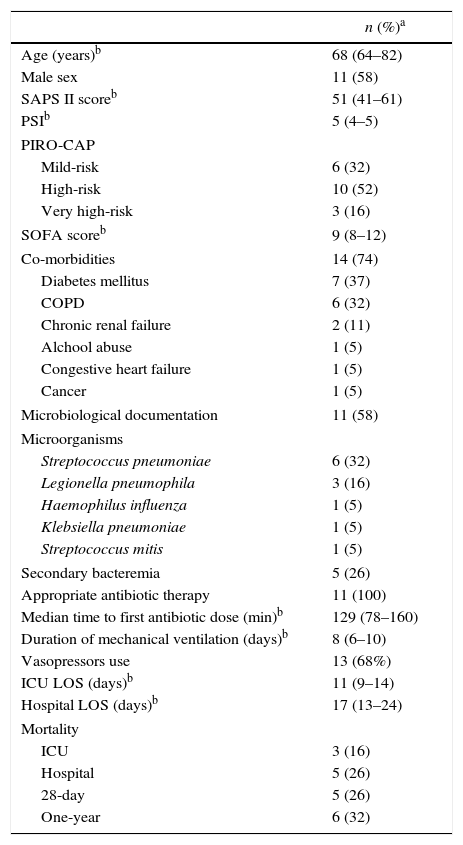
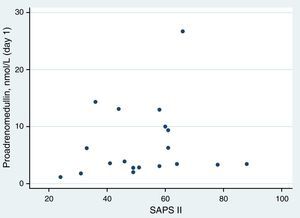
ResultsAt ICU admission median MR-proADM was 3.58nmol/l (IQR: 2.83–10.00). No significant association was found between its serum levels at admission and severity assessed by SAPS II (Spearman's correlation=0.24, p=0.31) or SOFA score (SOFA<10: <3.45nmol/l vs. SOFA≥10: 3.90nmol/l, p=0.74). Hospital and one-year mortality were 26% and 32%, respectively. No significant difference in median MR-proADM serum levels was found between survivors and non-survivors and its accuracy to predict hospital mortality was bad (aROC 0.53). After 48h of antibiotic therapy, MR-proADM decreased in all but 5 patients (median −20%; IQR −56% to +0.1%). Its kinetics measured by the percent change from baseline was a good predictor of clinical response (aROC 0.80). The best discrimination was achieved by classifying patients according to whether MR-proADM decreased or not within 48h. No decrease in MR-proADM serum levels significantly increased the chances of dying independently of general severity (SAPS II-adjusted OR 174; 95% CI 2–15,422; p=0.024).
ConclusionsIn SCAP patients, a decrease in MR-proADM serum levels in the first 48h after ICU admission was a good predictor of clinical response and better outcome.
Community-acquired pneumonia (CAP) remains a major cause of morbidity, mortality and healthcare costs.1–3 In 9–16% of cases, ICU admission is needed, due to severe respiratory failure, severe sepsis or septic shock.4–6 In these patients, mortality is high, reaching 50% in those patients requiring vasopressor support.7 Inadequate initial antibiotic therapy is a poor prognostic factor.8–10
Severity assessment and outcome prediction are fundamental in the management of CAP patients, both to allocate the most appropriate site of care and to select empirical antibiotic and adjuvant therapy. Response assessment is also paramount. Early identification of responders and non-responders could help reduce antibiotic consumption or allow earlier rescue antibiotic therapy. However, there is no standard definition for clinical response in severe CAP.
Efforts have been made to study the usefulness of biomarkers, as a complement to clinical judgment, in the diagnosis, severity assessment, treatment, prognosis and follow-up of CAP. In recent years, promising data regarding the role of adrenomedullin (ADM) in the management of CAP have been published.11–13 This hormone, a peptide with 52 aminoacids, is a potent vasodilator agent with immune modulating and metabolic properties and bactericidal activity that increases in sepsis.14 It is produced by multiple tissue types but, unfortunately, its serum levels are difficult to measure, since it is rapidly cleared from the circulation.15–17 However, the more stable mid-region fragment of proadrenomedullin (MR-proADM) directly reflects levels of ADM.18 This biomarker may be as good as validated pneumonia-specific severity scores at detecting patients with severe CAP and is probably better than other biomarkers, such as procalcitonin.11,12 MR-proADM serum level correlate well with mortality (both short and long term)19 and its addition to clinical scoring systems improves their discriminatory power.11–13
The purpose of our study was to evaluate the value of MR-proADM at ICU admission for further severity stratification and outcome prediction and to assess its kinetics as an early marker of response in SCAP patients.
Materials and methodsStudy designThis was a single-center, observational, prospective cohort study of 19 patients with severe CAP admitted to the Intensive Care Department of a tertiary care university hospital in Portugal between March 2012 and January 2013. The study was approved by the local ethics committee. Written informed consent was obtained from every patient or patient representative prior to inclusion in the study.
CAP was diagnosed when, in addition to suggestive clinical features (e.g. cough, fever, sputum production, pleuritic chest pain), a demonstrable infiltrate by chest radiograph or CT scan was present. Severe CAP was defined according to the Infectious Diseases Society of America/American Thoracic Society criteria (IDSA/ATS).20 In order to be included into this study, patients had to be older than 18 years old and have MR-proADM measured within 12h after the first antibiotic dose.
Data collectionThe following parameters were registered by the investigators in a specifically created database at the moment or within the first 24h of ICU admission: age, sex, co-morbidities, corticosteroids use, existence or development of septic shock and/or acute respiratory distress syndrome and empiric antibiotic therapy. The duration of mechanical ventilation, length of hospital and ICU stay and mortality (ICU, hospital and 1-year) were also recorded. Simplified Acute Physiology Score (SAPS) II21, Sepsis-related Organ Failure Assessment (SOFA) score22, Pneumonia Severity Index (PSI)6 and PIRO-CAP23 were calculated.
Proadrenomedullin determinationWithin 12h of the first antibiotic dose, a blood sample was withdrawn for the determination of MR-proADM and the process was repeated 48h later except for one patient.
MR-proADM concentrations (normal<0.52nmol/l) were measured at the Clinical Biochemistry Laboratory of Hospital Universitario Central de Asturias (Oviedo, Spain) in an automated Kryptor analyzer, using TRACE technology (Kryptor; BRAHMS, Hennigsdorf, Germany), without any pre-analytical treatments (i.e., extraction or derivatization). Analytical characteristics of the assay and reference values for the healthy adult population have already been described.24
Microbiologic evaluationAt the point of inclusion into the study, two pairs of blood cultures were collected. Blood cultures were processed using an automated microbiology growth and detection system (BACTEC). If there was bacterial growth, samples were Gram stained and subcultured. A bacteremic episode was defined as growth of a typical organism for CAP in at least one of four collected blood cultures.
Tracheal aspirate was taken from every patient whenever possible to test for bacteria according to standard procedures. Representative sputum originating from the lower respiratory tract was validated by the criteria of >25 granulocytes and <10 epithelial cells per low power field (total magnification×100).
Urine samples were collected and tested whenever possible for Legionella pneumophila and Streptococcus pneumoniae with an antigen test. Real-time polymerase chain reaction was used to evaluate the presence of respiratory virus in nasopharyngeal swab and bronchoalveolar lavage when clinically and epidemiologically indicated. Pleural fluid when available was also collected.
Identification of microorganisms and susceptibility testing was performed according to standard methods.
Statistical analysisCategorical variables are described as counts and percentages and continuous variables as the median and interquartile range (IQR). MR-proADM kinetics was quantified as the percent change from baseline according to: (100*(MR-proADM[48h]−MR-proADM[baseline])/MR-proADM[baseline]. Spearman's correlations were used to quantify the association between MR-proADM and severity scores. Median MD-proADM levels were compared across subgroups using the Kruskal–Wallis test. The area under the receiver operating characteristics (ROC) curves was estimated to quantify the discrimination of MR-proADM in predicting death at different times. The association between the change in MR-proADM and death was assessed by odds ratios (OR) estimated by logistic regression, adjusting for severity scores.
Statistical analysis was performed using the statistical package Stata version 11.1 for Windows (StataCorp LP, College Station, TX).
ResultsDemographic and clinical characteristics of patientsThe median age of the overall cohort was 68 years (IQR=64–82), 58% were male and 89.5% of the cases were in high-risk PSI classes IV and V. Median SAPS II and SOFA score were 55 (IQR=41–61) and 9 (IQR=8–12), respectively. Table 1 shows global baseline characteristics at hospital admission.
Main demographic and clinical characteristics of the study sample (n=19).
| n (%)a | |
|---|---|
| Age (years)b | 68 (64–82) |
| Male sex | 11 (58) |
| SAPS II scoreb | 51 (41–61) |
| PSIb | 5 (4–5) |
| PIRO-CAP | |
| Mild-risk | 6 (32) |
| High-risk | 10 (52) |
| Very high-risk | 3 (16) |
| SOFA scoreb | 9 (8–12) |
| Co-morbidities | 14 (74) |
| Diabetes mellitus | 7 (37) |
| COPD | 6 (32) |
| Chronic renal failure | 2 (11) |
| Alchool abuse | 1 (5) |
| Congestive heart failure | 1 (5) |
| Cancer | 1 (5) |
| Microbiological documentation | 11 (58) |
| Microorganisms | |
| Streptococcus pneumoniae | 6 (32) |
| Legionella pneumophila | 3 (16) |
| Haemophilus influenza | 1 (5) |
| Klebsiella pneumoniae | 1 (5) |
| Streptococcus mitis | 1 (5) |
| Secondary bacteremia | 5 (26) |
| Appropriate antibiotic therapy | 11 (100) |
| Median time to first antibiotic dose (min)b | 129 (78–160) |
| Duration of mechanical ventilation (days)b | 8 (6–10) |
| Vasopressors use | 13 (68%) |
| ICU LOS (days)b | 11 (9–14) |
| Hospital LOS (days)b | 17 (13–24) |
| Mortality | |
| ICU | 3 (16) |
| Hospital | 5 (26) |
| 28-day | 5 (26) |
| One-year | 6 (32) |
SAPS: Simplified Acute Physiology Score; PSI: Pneumonia Severity Index; PIRO-CAP: Predisposition, Infection, Response, Organ dysfunction-Community-acquired pneumonia; SOFA; Sepsis-related Organ Failure Assessment; COPD: Chronic Obstructive Pulmonary Disease; IDSA/ATS: Infectious Diseases Society of America/American Thoracic Society; ICU: Intensive Care Unit; LOS: Length of stay.
SCAP was microbiologically documented in 58% cases and S. pneumoniae (n=6) was the leading pathogen followed by L. pneumophila (n=3). All patients received antibiotic therapy concordant with IDSA/ATS guidelines.20
Proadrenomedullin at ICU admission and severity scoresMedian MR-proADM at ICU admission was 3.58nmol/l (IQR=2.83–10.00). In this cohort, no significant association was found between MR-proADM serum levels at admission and severity assessed by SAPS II (Spearman's correlation=0.24; p=0.31) (Fig. 1). MR-proADM serum levels were higher in patients with SOFA score ≥10, but this difference did not reach statistical significance (SOFA score≥10: 3.90nmol/l vs. SOFA score<10: 3.45nmol/l; p=0.74) (Fig. 2).
As for SOFA score, patients with higher pneumonia-specific severity scores, such as PSI (class III and IV: 2.78nmol/l vs. class V: 3.74nmol/l; p=0.29) and PIRO CAP (mild risk: 2.53nmol/l vs. high risk: 4.93nmol/l vs. very high risk: 3.89; p=0.23), had higher MR-proADM serum levels but the differences were not statistically significant.
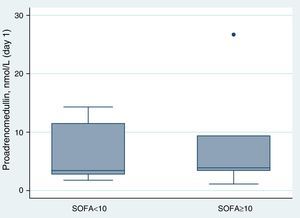
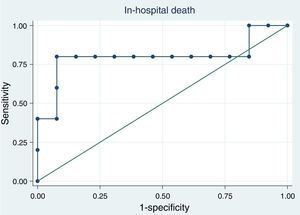
Proadrenomedullin at ICU admission and outcomeICU, hospital and one-year mortality were 16%, 26% and 32%, respectively. Median MR-proADM serum levels were similar, in survivors and non-survivors regarding hospital [survivors 3.51nmol/l (IQR=2.60–13.00) vs. non-survivors 3.89nmol/l (IQR=3.10–8.10); p=0.89] and 1-year mortality [survivors 3.58nmol/l (IQR=2.40–13.00) vs. non-survivors 3.67nmol/l (IQR=3.30–7.20); p=1.0]. Receiver operating characteristic (ROC) curve analysis showed that this biomarker at ICU admission had a bad discriminatory power to predict hospital [aROC 0.53; 95% confidence interval (CI) 0.26–0.79] and 1-year mortality (aROC 0.51; 95% CI 0.25–0.78) (Fig. 3).
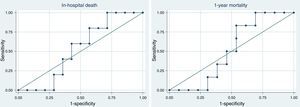
Proadrenomedullin kinetics and outcomeAfter 48h of antibiotic therapy, MR-proADM serum levels decreased in all but 5 patients (median −20%; IQR=−56% to +0.1%). MR-proADM kinetics measured by the percent change from baseline was a good predictor of hospital mortality (aROC 0.80; 95% CI: 0.47–1.00) (Fig. 4).
The best discrimination was achieved by classifying patients according to MR-proADM decreasing (one patient died out of 13) or not (4 out of 5 died) within 48h of antibiotic therapy. The absence of reduction in this biomarker significantly increased the chances of dying in the hospital independently of general severity [SAPS II-adjusted odds ratio (OR) 174; 95% CI: 2–15,422; p=0.024].
DiscussionIn our study MR-proADM at ICU admission and within 12h of first antibiotic dose did not further stratify severity in patients with SCAP. Furthermore, serum MR-proADM at this stage did not perform well as a predictor of both short- (ICU and hospital) and long-term (one-year) mortality. Nevertheless, MR-proADM kinetics in the first 48h after antibiotic therapy was a good tool to predict hospital mortality.
Considering the morbidity and mortality associated with CAP, early identification of high risk patients is of paramount importance. Several papers demonstrate an association between this biomarker's serum levels and severity assessed by pneumonia specific scores such as PSI. Christ-Cain et al. and Huang DT et al.,11,12 reported that MR-proADM levels consistently rise as PSI class increases (p<0.001). In 49 patients with severe sepsis/septic shock due to CAP admitted to the ICU, MR-proADM consistently rose as PSI advanced from II to V (p=0.02).25 Yet, our results, like those of Akpinar et al.,26, do not support this finding. Although patients in PSI class V presented higher MR-proADM serum levels, we did not observe any significant difference across different risk classes of this score. Besides the sample size, differences in study cohort, namely the fact that only severe CAP patients were included, may explain these different results.
We also did not find a significant association with severity assessed by SAPS II or SOFA score. In contrast, Marino et al.,27, in a prospective observational study in patients with suspected sepsis presenting to the Emergency Department, observed a moderate association of MR-proADM with severity of disease evaluated by APACHE II score (r=0.46; p<0.001). This correlation was also described by Travaglino et al.28 In a Swiss study with 101 critically ill patients, MR-proADM levels on ICU admission exhibited correlation with APACHE II score (r=0.42; p<0.001) and SAPS II score (r=0.5; p<0.001).29 These differences may be explained by the different types of population studied. Indeed, our study was the first to include only critically ill patients with severe sepsis or septic shock, a significant proportion of them (68%) needing vasopressors at ICU admission. The fact that MR-proADM serum levels are higher in these groups of patients27,29 may explain the lack of relationship between this biomarker and severity scores in our study.
In the emergency department, MR-proADM seems to be a very good prognostic tool to predict both short and long-term outcome in patients with lower respiratory tract infections.19 However, when the analysis is restricted to the most severe patients, conflicting results have been published. According to Christ-Crain study,29 the prognostic accuracy for ICU mortality of this biomarker on admission, in septic critically ill patients, is good (AUC 0.81), similar to severity scores such as APACHE II and SAPS II score and significantly better than other biomarkers such as C-reactive protein. Huang's study12 showed that, within PSI classes IV/V (n=546), subjects with MR-proADM higher than 1.45nmol/l had a higher 30-day mortality rate (23% vs. 9%; p<0.001). In a subgroup of patients (n=61) with high risk PSI scores (classes IV and V), Courtais et al.,30 showed that, in univariate logistic regression analysis, MR-proADM levels significantly predicted 30-day mortality risk (OR 4.68; 95% CI: 1.66–20.22) with an aROC curve of 0.81 (95% CI: 0.65–0.96). Nevertheless, only 24 of these patients were admitted to the ICU.
Like other studies, we observed a low discriminatory power of MR-proADM to predict hospital and one-year mortality. Apkinar26 reported that this biomarker had a bad discriminatory power to predict 4- (AUC 0.505) and 8-week mortality (AUC 0.513) and Marino et al.27 showed a poor performance to predict 28 day-mortality (AUC 0.60). Despite being slightly better than previously reported, in Suberviola's study the discriminatory power of this pro-hormone to predict hospital mortality was only moderate (AUC 0.72).25
Until now, there have been no published data regarding the value of MR-proADM kinetics in the management of SCAP patients. In our cohort, we found that after 48h of antibiotic therapy, the percent change from baseline was a good predictor of hospital mortality. Indeed, the absence of decrease in serum levels was an independent risk factor for hospital mortality. Similar findings were described in other groups of patients. For instance, in febrile patients with hematologic malignancies,31 serum MR-proADM levels dropped in patients who responded to therapy whereas in patients who did not respond there was no significant change between initial (at fever onset) and follow-up levels (4–7 days later). Furthermore, septic patients admitted to the emergency department with high plasma ADM (>70pg/ml) that present a decrease in serum levels in the first 4 days of therapy have a higher survival rate than those patients whose levels remain above that threshold (100% vs. 36%).27
The fact that this biomarker was collected within 12h after the first antibiotic dose in patients without prior antibiotic use and that all patients were prospectively enrolled are two of the strengths of this study. However, some limitations also merit consideration, namely the fact that it was a single center study and that the generalizability of our findings is limited by the small sample size (only 19 patients). Therefore, these results should be validated in a large multicenter study.
ConclusionsWe concluded that, in SCAP patients, MR-proADM on ICU admission is not useful for further severity stratification and to predict both short- and long-term outcome. However, its kinetics in the first 48h after antibiotic therapy may be a helpful tool to assist clinicians in identifying patients with a better clinical outcome. Moreover, although further studies are needed, decreasing levels of this biomarker, as a sign of early response to therapy, may lead to shorter duration of antibiotic therapy and play a role in antibiotic stewardship.
Authors’ contributionsAll authors have made substantial contribution to the conception and design of the study as well as in the drafting, revising and final approval of the version to be published. JMP and AA performed statistical analysis.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki). The authors have obtained the written informed consent of the patients included in this study. The corresponding author is in possession of this document.
Conflict of interestThe authors declare that they have no competing interests.