Tuberculosis remains a major public health problem worldwide. HIV co-infection is contributing to an increased incidence of the disease, particularly that caused by multidrug resistant strains of Mycobacterium tuberculosis (MT). We describe an HIV-infected patient with pleural and lymph node tuberculosis diagnosed by pleural effusion characteristics and biopsy specimens, without MT identification, that further presented with knee-joint involvement. Arthrocentesis allowed MT isolation and drug susceptibility testing, resulting in a diagnosis of multidrug-resistant tuberculosis and an appropriate treatment regimen.
MT identification and drug susceptibility tests are very important, especially for HIV co-infected patients.
A tuberculose constitui um importante problema de saúde pública mundial. A co-infecção pelo HIV contribui para o aumento da incidência da doença e em particular a causada por estirpes de Mycobacterium tuberculosis (MT) multirresistentes. Os autores descrevem um doente HIV-positivo com tuberculose pleural e ganglionar diagnosticada pelas características bioquímicas do líquido pleural e resultados anatomo-patológicos de biopsias mas sem identificação do agente, que posteriormente apresentou envolvimento do joelho. A artrocentese do joelho permitiu o isolamento do MT e a realização de teste de sensibilidade possibilitando o diagnóstico de tuberculose multirresistente e a instituição de um esquema terapêutico adequado.
A identificação do MT e a realização de testes de sensibilidade são muito importantes, especialmente em doentes com co-infecção por HIV.
Tuberculosis (TB) remains a major public health problem worldwide. It has been estimated that 14 million people were living with TB in 2009, and that 1.7 million have died from this disease, among whom 0.38 million were seropositive for the human immunodeficiency virus (HIV).1 HIV infection is not only associated with an increased incidence of TB but also an increased incidence of disease caused by multidrug-resistant (MDR) strains of Mycobacterium tuberculosis (MT).2 As immunocompetence decreases in HIV-infected patients, the incidence of atypical presentations increases, including high proportions of patients with extrapulmonary disease and disseminated TB.3,4
Although, osteoarticular TB is reported in 1–3% of patients with TB,5,6 knee-joint involvement of TB is extremely rare, present in only about 0.1% of patients.7 Unlike all other forms of extrapulmonary manifestations that are more common in HIV-1, extraspinal tuberculous arthritis appears to occur even less frequently.8
Non-HIV patients previously treated for TB are also at increased risk of developing drug resistance. MDR is the most critical form of drug-resistant bacteria because it makes therapeutic regimens containing first-line drugs much less effective.9 Furthermore, if MDR is not promptly recognized, resistance can be further amplified.10 Thus, microbiological diagnosis and identification of MDR using drug susceptibility test (DST) have been recommended.11,12 Identification of MDR can allow initiation of treatment with second-line drugs, providing a better chance of cure and preventing the development and spread of further resistance.
To reduce MDR-TB incidence in Portugal, by early recognition of resistance and by adequate treatment, reference centers were established. Whenever a MDR strain of M. tuberculosis is detected the center within that geographic area is notified. If necessary, current medication is optimized or a new treatment regimen is started.
Case reportIn May 2009, a 40-year-old male patient presented having had a persistent fever for 5 days and a 2-month history of anorexia and weight loss (12kg). He had no dyspnea, cough or sputum. Examination of his medical records showed that he had a history of alcoholism and drug abuse and HIV-infection since 1993 (taking antiretroviral therapy (ART) since 2009). In 2001, he was diagnosed with pulmonary and lymph node tuberculosis. At that time, he had an isoniazid (H) and streptomycin (S) resistant strain and completed 16 months of treatment with H, rifampicin (R), pyrazinamide (Z) and ethambutol (E)/HR.
General examination at the hospital (May 2009) revealed a poorly built, malnourished man weighing 45kg. He was febrile (auricular temperature 39.5°C), tachycardic and normotensive. Pulse oximetry (FiO2 21%) was 99% and chest auscultation revealed decreased breath sound in the lower-left lung field.
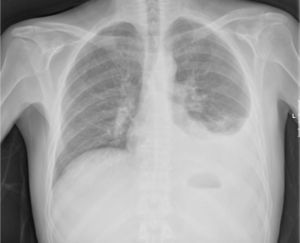
Laboratory findings on admission revealed a normal WBC count (CD4+-269mm3), a low hemoglobin concentration (11.9g/dL) and an increased C-reactive protein concentration (24mg/dL). Chest X-rays showed the presence of left pleural effusion (Fig. 1). A thoracic-abdominal computed tomographic scan revealed no lung parenchymal abnormalities but a mesenteric mass with 5.5cm in diameter.
A diagnostic thoracentesis was performed revealing an exudate with a preponderance of lymphocytes and an elevated adenosine deaminase (ADA) concentration (63U/L). A biopsy of the mesenteric mass showed epithelioid granulomas, smear and culture of this specimen were negative. Sputum and pleural fluid smears and cultures were persistently negative.
These findings suggested pleural and lymph node TB. Despite the past DST (2001) the patient began antituberculosis therapy with HRE. After 2 weeks, however, the fever persisted and he developed pain and swelling of his right knee, with limited joint mobility. An arthrocentesis was performed for both diagnosis and symptom relief, and synovial fluid was collected. Smears of the fluid were negative for acid-fast bacilli, but cultures were positive. Molecular DST showed a MDR strain of MT with resistance to H and R. Second-line DST showed that the MDR strain was susceptible to ethambutol, capreomycin, moxifloxacin, ethionamide and cycloserine.
Following the diagnosis of MDR-TB in July 2009, our reference center was alerted. The patient was started on a new therapeutic regimen, consisting of ethambutol, capreomycin, moxifloxacin, ethionamide and cycloserine.
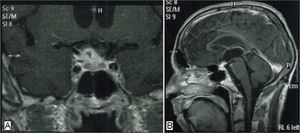
After 3 months of therapy, the patient had a convulsion. Cycloserine toxicity was suspected and treatment with this drug was suspended. Cerebral magnetic resonance imaging showed probable cerebral tuberculous granulomas (Fig. 2). Steroids were therefore added to his treatment regimen.
After 6 months of treatment, capreomycin treatment was halted and the maintenance phase of treatment began. It was concluded after a total of 18 months, by which time there was clear evidence of recovery, regression of the cerebral lesions, complete resolution of knee symptoms and persistent improvement of the pleural effusion.
DiscussionWe describe a patient with MDR-TB who was diagnosed by mycobacteriological analysis of synovial fluid of the knee. Although previous reports of tuberculous synovitis (particularly in HIV patients) have been described in literature,13–15 to our knowledge this is the first time that the diagnosis of a MDR-TB strain in synovial fluid of the knee has been described.
Knee-joint involvement is a relatively rare manifestation of extrapulmonary TB, but the number of patients is increasing.8 This presentation is particularly associated with HIV infection,3 usually as a result of hematogenous spread from the primary focus.
This HIV-patient presented with persisting fever, pain and swelling of his right knee, with limited joint mobility after 2 weeks of a probable diagnosis of pleural and lymph node TB, making infection the most likely diagnosis, specifically a manifestation of extra-pulmonary TB. Although it was this previous diagnosis that prevented the diagnostic delay often associated with osteoarticular TB,7 careful investigation should be performed as other infection agents might be responsible and, as shown in this case, the isolation of infectious agent might have enormous impact on the treatment and final outcome. A bursitis due to Mycobacterium avium complex in an immunocompetent patient16 has already been described and it also led to a granulomatous response. Although nontuberculous mycobacteria infections could explain the epithelioid granulomas, the osteoarticular, pleural and lymph node involvement found in this patient are not common manifestations of those infections.17
Although 40–60% of patients with disseminated TB also have pulmonary TB,18 the thorax CT scan in this patient showed no lung parenchyma involvement, and sputum smears were persistently negative. Also interesting were the pleural fluid smear and culture negative results. According to the literature not only is pleural effusion more common in HIV-patients19 but also, the profitability of direct examination and culture of pleural fluid in those patients is higher.19,20 One could argue that the presented patient had been under ART since 2009 and had a CD4 counts >200cells/mm3, nevertheless this should have been noticed. Although pleural histological examination and microbiologic analysis is known to be profitable it was not performed.
This patient had pulmonary and lymph node tuberculosis in 2001. Whether recurrent tuberculosis is a reactivation or a re-infection is still a controversial question.21 Recently Reniero et al. found that there was substantial evidence – both experimental and epidemiological – to support the role of exogenous re-infection in tuberculosis.22 Regarding the presented case, one could only know for sure if M. tuberculosis genotyping had been performed, but we should note the fact the he was not properly treated in 2001 for a isoniazid resistant strain. The patient underwent maintenance therapy period with rifampicin and isoniazid (to which he was known to be resistant) and eight years later was diagnosed with a M. tuberculosis strain resistant to both of those drugs.
Drug resistance may be due to the administration of improper treatment regimens by healthcare workers and failure to ensure that patients complete the whole course of treatment.12 Prior treatment for TB is the most critical patient-specific risk factor for MRD-TB23,24 as well as HIV infection that has been associated with raised MDR levels.12 Our patient had both of these risk factors for MDR.
To determine the optimal treatment regimen, specimens should be obtained for culture and DST analysis. In our patient, however, sputum and pleural fluid cultures were negative, whereas synovial fluid was positive, allowing only the latter to be analyzed. Our findings highlight the importance of identifying a MDR strain, especially in a patient with disseminated TB and cerebral tuberculomas and also stress the importance of a MDR-TB treatment component integrating the national programme of TB management.
The CRRMR ensures that the most appropriate treatment is selected by experts in programmatic management of drug-resistant TB. It also ensures that drugs to treat drug-resistant TB are available and that domiciliary DOT is strictly performed. In the present case, the CRRMR was notified by the microbiology laboratory only when the results from the fluid analysis were known. Although this reference approach enables the center physicians to suggest a treatment approach in cases of suspected resistant strains (making it difficult to justify the HRE regiment chosen) until diagnosis confirmation, it also ensures that action is taken after the results are known (which did not occur in 2001 when the patient had a HS-resistant tuberculosis).
Monthly evaluations at the reference center allow for rapid intervention in case of clinical deterioration and strict monitoring of adverse effects caused by second-line drugs. Cerebral tuberculomas were diagnosed following the investigation of a convulsion episode that occurred despite 3 months of a correct treatment regimen and was confirmed not only by drug susceptibility tests but also by the patient's final outcome. It is the authors’ belief that the cerebral involvement was probably already present at the time of diagnosis although asymptomatic and that cycloserine, known to increase the brain's general reactivity led it to become symptomatic.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: van Zeller, M, et al. Tuberculose multirresistente diagnosticada através de análise de líquido sinovial. Rev Port Pneumol. 2012. doi:10.1016/j.rppneu.2012.02.002.