Activating mutations in EGFR are important markers of response to TKI therapy in mNSCLC.1,2 In robust phase 3 trials, erlotinib (E) has been shown to improve progression free survival (PFS), compared with chemotherapy (CT), in first-line treatment (1L), patients with mNSCLC EGFR MUT+, ORR – 58%, PFS – 9.7 months and overall survival (OS) 22.9 months in caucasions.1,2 MuTAR study, assessed the efficacy of 1L Erlotinib in portuguese pts.
We undertook this open-label multicenter, phase II trial, at 9 hospitals in Portugal. Eligible patients included adult patients with histologically or cytological documented inoperable mNSCLC EGFR MUT+ with no history of chemotherapy or therapy against metastatic disease.
Treatment consisted of Erlotinib 150mg/day per os, in tablet form, until disease progression, unacceptable toxicity, death or withdrawal of consent.
The primary efficacy endpoint was objective response rate (ORR), defined as complete or partial response according to RECIST version 1.1.
An interim analysis was conducted with a cut-off date on the 30th September 2013, to analyse preliminary clinical benefits and compare it with the available literature. At the time of interim analysis, 3 patients were still in treatment.
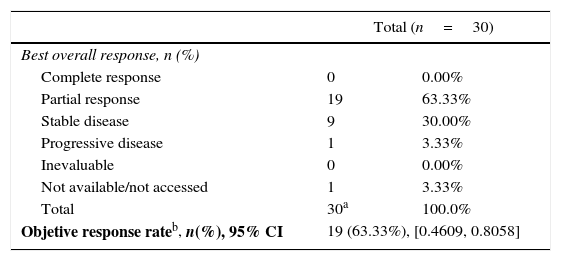
The recruitment period was between February 2011 and March 2012. Of the 216 screened patients, 205 performed EGFR mutation test, with 38 showing activating EGFR mutations and 30 (14.6%) were eligible to be included in the study (44.7% with exon 19 and 55.3% with exon 21). The ORR (ITT population) was 63.3% (95%CI: 46.1%–80.6%) (Table 1). The median PFS and overall survival (OS) (ITT population) were 10 months (95%CI: 7.7–16) and 20.7 months respectively. A total of 340 adverse events (AE) were observed in 29 patients;
Best overall response: RECIST criteria – ITT population.
In conclusion, E in 1L treatment of mNSCLC EGFR Mut+pts showed similar results compared with other clinical trials in Caucasian patients regarding ORR, PFS and OS. First-line E was effective and well tolerated in Portuguese EGFR Mut+patients. Our findings strengthen also the rationale for routine baseline assessment of EGFR mutation status in pts with mNSCLC and for treatment benefit of mutation positive patients with EGFR TKIs.
AuthorshipAll the authors approved the final draft of the manuscript for submission. Dr. Fernando Barata conceived this study and supervised all aspects of its implementation. All the authors contributed to data collection and the interpretation of the results and the proof reading of the manuscript.
Conflict of interestThe authors declare no conflict of interest.
This work was sponsored by Roche Farmacêutica Química, Lda and was presented in 6o Congresso Português do Cancro do Pulmão, October 10th 2014.