Tuberculosis (TB) is the ninth cause of global death, more than any other infectious disease. With growing drug resistance the epidemic remains and will require significant attention and investment for the elimination of this disease to occur. With susceptible TB treatment not changing over the last four decades and the advent of drug resistance, new drugs and regimens are required.
Recently, through greater collaboration and research networks some progress with significant advances has taken place, not withstanding the comparatively low amount of resources invested. Of late the availability of the new drugs bedaquiline, delamanid and repurposed drugs linezolid, clofazimine and carbapenems are being used more frequently in drug-resistant TB regimens.
The WHO shorter multidrug-resistant tuberculosis regimen promises to reach more patients and treat them more quickly and more cheaply.
With this new enthusiasm and hope we this review gives an update on the new drugs and perspectives for the treatment of drug-susceptible and drug-resistant tuberculosis.
Effective treatment of tuberculosis (TB) is reliant on several bactericidal and sterilising drugs administered in combination for an adequate duration, to guarantee antimicrobial efficacy while preventing selection of drug-resistant mutants and achieve permanent cure.1 Current treatment regimens are, however, unsatisfactory due to low efficacy, high toxicity, long duration and significant health resource burden with treatment for multidrug-resistant TB (MDR-TB defined as resistance to isoniazid and rifampicin); drug–drug interactions are also notable as exemplified by rifampicin with protease inhibitors and other antiretrovirals (ARVs). Some combinations include drugs that have been registered for indications other than TB and are therefore repurposed and used ‘off-label,’ such as oxazolidinones, carbapenems, or clofazimine, for the treatment of highly-resistant TB cases.2,3 In May 2016, WHO issued guidance that people with TB resistant to rifampicin, with or without resistance to other drugs, should be treated with an MDR-TB treatment regimen.4 Despite recent advances, TB including drug-resistant forms, continues to present a number of challenges to physicians and national TB programmes. With 10.4 million infections in 2015 TB was one of the top 10 causes of death worldwide killing 1.8 million people, ranking above HIV/AIDS as the leading cause of death from an infectious disease and one of the major global health problems.5 This is despite the fact that with a timely diagnosis and correct treatment, the majority of people developing TB disease can be cured. Global progress depends on significant advances in TB prevention and care. Worldwide, the rate of decline in TB incidence remained at only 1.5% from 2014 to 2015. This needs to accelerate to a 4–5% annual decline by 2020 to reach the first milestones of the End TB Strategy.6 New TB drugs and regimens are urgently needed to improve cure rates for people with drug-resistant TB (currently around 50% globally) and to shorten the treatment of both drug- susceptible and drug-resistant TB (currently at least six and at least nine months respectively). For the first time in almost four decades, two new TB drugs, bedaquiline, and delamanid, have become available. These are recommended by the World Health Organisation (WHO) for the treatment of drug-resistant TB under certain conditions.4 These drugs have, however, been tested for efficacy as add-ons to the conventional (or longer) WHO-recommended treatment regimen for MDR-TB, their use in combination and with repurposed drugs are still under study, it is hoped that these new regimens will lead to increased treatment efficacy treatment duration at the same time improving safety.7
However, the development of new, efficacious combination regimens for TB treatment is lengthy and costly. Under our current modus operandi, if new drugs were added or substituted into existing regimens one at a time, it would take 20–30 years to develop a new regimen of three to four new drugs.8 Developing a regimen without the need to obtain individual drug approvals separately before testing novel combinations would substantially reduce both the duration of the regimen development pathway and the expenditure required to make progress.
Development of shorter, simpler regimens combining new and existing drugs requires detailed information on their respective safety and toxicity; their potential for drug–drug interactions (DDIs); their propensity for development of drug resistance while on therapy; and their use in specific patient populations such as persons infected with human immunodeficiency virus (HIV), pregnant women, and children. The development of target product profiles recently published by WHO allows the identification of desired product attributes or priorities to be considered during the product development process; expanding on this, the determination of target profiles for TB treatment regimens (i.e., target regimen profiles).9
Current treatment for drug-susceptible TB consists of the standard four-drug regimen comprising, isoniazid (H), rifampicin (R), ethambutol (E) and pyrazinamide (Z) for six months, (2HREZ/4HR). It has shown high efficacy in achieving cure rates around 90–95%, both in treatment under the oversight of TB control programmes and trial conditions.10,11 While the regimen is effective if taken as prescribed, patients struggle to take their medication daily over six months. Moreover, the regimen is not always well-tolerated, dosed, absorbed and the regimen may give side effects and severe adverse events like hepatotoxicity. Problems with compliance, sub-optimal drug levels and tolerability can lead to resistance. More selective, better-tolerated and shorter drug regimens are needed to eliminate TB more quickly and efficiently. Programmatic failures, conflict and lack of resources have facilitated the burgeoning of drug resistance defined as MDR/XDR-TB (extensively drug-resistant TB defined, as resistance to isoniazid and rifampicin, and to at least one fluoroquinolone and one injectable second-line anti-TB drug in addition to isoniazid and rifampicin) are both clinical and public health priorities), which has now reached critical levels becoming an emergency. Drug-resistant TB is a harbinger for worse outcomes.12 Shorter and more tolerated regimens are desperately needed to increase adherence, reduce loss to follow-up and treat both susceptible, non-resistant and MDR/XDR-TB allowing for diagnostic and management simplification.13 Treatment of MDR-TB requires longer course duration, more expensive drugs with a higher toxicity profile, compared with treatment of drug-susceptible TB. The requirement of several antimicrobials and the extended treatment period complicate the ability to administer, manage and follow patients who frequently are not able to comply or tolerate treatment. Moreover, the accessibility of drug susceptibility testing and effective treatment regimens for MDR and XDR-TB further complicate matters. Outcomes for MDR-TB are still dire, with only 52% of the MDR/RR-TB (rifampicin-resistant TB) patients who started treatment in 2013 being successfully treated, 17% of patients died, and 9% of patients had treatment failure (22% were lost to follow-up or not evaluated). The treatment success rate in XDR-TB patients was only 26%.5 Individualised treatment regimens having a higher treatment success than standardised regimens but these tend to cost more and require availability of drugs and diagnostic capacity.14 Moreover, greater drug resistance beyond MDR-TB results in worse outcomes.15,16
AimThe review aims to report what is new in the treatment of drug-susceptible and drug-resistant tuberculosis. This manuscript is split into sections summarising further evidence on current, repurposed drugs, new compounds as well news on trials and new perspectives.
MethodsA non-systematic literature review based on a PubMed search using specific keywords, including various combinations of TB, susceptible, resistant, MDR-TB, XDR-TB, clinical trial was performed. References of the most relevant papers were retrieved to improve the search sensitivity. Due to differences between studies, data were combined as a narrative synthesis. Manuscripts written in English were considered.
DiscussionA short discussion of current recommendations for treatment of drug-susceptible and drug-resistant tuberculosis is offered here, but may be followed in greater depth elsewhere.7,11,17,18
Treatment of drug-susceptible tuberculosisThe preferred regimen in patients without suspected resistance consists of a two-month intensive phase with daily HRZE, followed by a 4-month continuation phase of HR. Ethambutol can be discontinued if susceptibility test show patient's isolate is susceptible to HR. However, if the patient's chest radiography shows cavitation, and the culture is still positive at completion of the initial phase, the opinion is to extend the continuation phase for an additional 3 months.7,11
Even though the current regimen has proved efficacious over the last 30 years, shorter and simple anti-TB regimens are required and expected to increase patient adherence and therefore disease control. Several studies have demonstrated fluoroquinolones bactericidal and sterilising activity TB, their ability to shorten the current treatment regime was recently evaluated. The shorter 4 months FQ-containing regimens (Remox, Oflotub and Rifaquin) were associated with significantly higher rates of relapse at 18 months follow-up compared with the standard 6-month rifampicin-containing regimen, even though at 2 months the FQ-containing regimens had slightly higher rates of culture conversion (not statistically significant)19–22 these regimens are not recommended.11 Four-month standard regimens are so far only recommended in the ATS guidelines for minimal disease, sputum smear, and culture negative cases.7 TBTC study 31, Rifashort and Stand trials are pursuing the idea of shortening current pan sensitive TB regimen, these are evaluating the utility of rifapentine, high dose of rifampicin and a complete new regimen including new drugs.
Therapeutic drug monitoring (TDM) is useful in the personalisation of TB treatment allowing for confirmation of adequate levels or to warn the clinician of sub-therapeutic levels which may proffer resistance or supra-therapeutic levels which may lead to toxicity. TDM is particularly useful in the setting of severe gastrointestinal abnormalities: severe gastroparesis, short bowel syndrome, chronic diarrhoea with malabsorption, when there is potential for drug–drug interactions, impaired renal clearance: renal insufficiency, peritoneal dialysis, critically ill patients (TB meningitis), HIV infection, diabetes mellitus and treatment using second-line drugs.23,24
Treatment of drug-resistant tuberculosisThe World Health Organisation (WHO) has recently updated the classification of new anti-tuberculosis drugs.4,25 Previous World Health Organisation (WHO) guidelines classified anti-TB drugs into five main groups, based on safety and effectiveness considerations. This classification originated in 2006, updated in 2008, 2011 and, finally, in 2016 based on new evidence, mainly from the former group 5 drugs.26–29
Current guidelines recommend that patients with RR or MDR- TB, not eligible for the shorter regimen, should receive at least five active TB medicines during the intensive phase, including pyrazinamide and four core second-line TB medicines – one is chosen from group A, one from group B, and at least two from group C. If the minimum of effective TB medicines cannot be composed as above, an agent from group D2 and other agents from D3 may be added to bring the total to five. Table 1 depicts the current WHO recommended classification for building an MDR-TB regimen.
WHO categorisation of second-line antituberculosis drugs recommended for the treatment of rifampicin-resistant and multidrug-resistant tuberculosis.4
| Group A: fluoroquinolones |
| • Levofloxacin |
| • Moxifloxacin |
| • Gatifloxacin |
| Group B: second-line injectable agents |
| • Amikacin |
| • Capreomycin |
| • Kanamycin |
| • (Streptomycin) |
| Group C: other core second-line agents |
| • Ethionamide/prothionamide |
| • Cycloserine/terizidone |
| • Linezolid |
| • Clofazimine |
| Group D: add-on agents (not part of the core multidrug-resistant tuberculosis regimen) |
| D1 |
| • Pyrazinamide |
| • Ethambutol |
| • High-dose isoniazid |
| D2 |
| • Bedaquiline |
| • Delamanid |
| D3 |
| • Para-aminosalicylic acid |
| • Imipenem plus cilastatin (requires clavulanate) |
| • Meropenem (requires clavulanate) |
| • Amoxicillin plus clavulanate |
| • (Thioacetazone)a |
Should the shorter regimen not be indicated due to known or suspected resistance to one or more agents in the shorter regimen or intolerance, a longer regimen with at least four active drugs should be used (not counting ethambutol or pyrazinamide). According to WHO MDR TB guidelines this regimen should include if possible: Pyrazinamide, Moxifloxacin, a second line injectable, two core second-line agents (ethionamide/prothionamide, cycloserine, linezolid and/or clofazimine). If a regimen cannot be made with the above agents, further additional agents may be used to build a regimen; these include bedaquiline, delamanid, p-aminosalicylic acid, imipenem/meropenem, amoxicillin-clavulanate.
Expert consultation of these challenging cases with regional, national or WHO/ERS Consilium is recommended.30
Shorter regimens for MDR-TBA 9–12-month standardised regimen is recommended by WHO for all patients with pulmonary MDR/RR-TB (excluding pregnant women, extrapulmonary TB and some other exclusions) that is susceptible to fluoroquinolones and aminoglycosides not previously treated with second-line agents.5 This regimen consists of an intensive phase with gatifloxacin/moxifloxacin, kanamycin/amikacin, ethionamide/prothionamide, clofazimine, high dose or 10mg/kg isoniazid (max 600mg a day), ethambutol and pyrazinamide for 4–6 months, followed by a continuation phase of 5 months with gatifloxacin/moxifloxacin, clofazimine, ethambutol, and pyrazinamide.19–21 However, the appropriate management of such regimens is essential in order not to select for further resistance; adequate drug susceptibility testing should be provided for all cases, M/XDR-TB case management to highly experienced clinicians based on international guidelines is recommended. All these agents require a careful management in the context of individualised regimens under close clinical and laboratory monitoring.30,31
The first evidence of utility of the shorter regimen was from an observational study evaluating the effectiveness of 6 standardised regimens for patients with proven MDR-TB previously untreated with second-line drugs. The most effective treatment regimen “Bangladesh” standardised regimen, achieved a relapse-free cure of 87.9% among 206 patients with a 9 to 12-month duration of therapy with clofazimine, gatifloxacin, ethambutol and pyrazinamide throughout the treatment period and including prothionamide, kanamycin and high-dose isoniazid during an intensive phase of a minimum of 4 months, this regimen achieved < 1% failure and 90% relapse-free cure.32 Moreover, an update of this study has shown that 84.4% of the 515 patients (enrolled from 2005 to 2011) had a bacteriologically favourable outcome.33 A recent, meta-analyses reported that shorter regimens were effective in treating MDR-TB; however, the authors stated there was uncertainty surrounding the generalisability of the high rate of treatment success to less selected populations, to programmatic settings and in the absence of drug susceptibility tests to crucial component drugs which is essential as failure/relapse was associated with fluoroquinolone resistance with an OR of 46.13
Experience with the use of the shorter MDR-TB regimen still remains limited, and is conditionally recommended for MDR/RR-TB patients under specific eligibility criteria. The ongoing STREAM-1 trial initiated in 2012 is evaluating the efficacy and safety of this regimen, results are expected in 2018, preliminary results suggests failure at demonstrating non-inferiority, however it is a good option for selected patients. Whether bedaquiline, could play a role in a shorter regimen is still under evaluation in the phase 2 STREAM trial.
Repurposed drugsWhilst awaiting new drugs and regimens, repurposing of drugs licensed for other conditions has been used to treat MDR/XDR-TB in the interim period.34 Fluoroquinolones, kanamycin, amikacin, clofazimine, linezolid, carbapenems, amoxicillin/clavulanic acid are all repurposed medications. Third and fourth generation fluoroquinolones (levofloxacin, moxifloxacin and gatifloxacin) are frequently used for the treatment of isoniazid-monoresistant TB and MDR-TB where it forms the most important component of a second-line regimen.35,15 Fluoroquinolones were hypothesised to have potential in reducing the duration of therapy in TB. However, three recently concluded clinical trials have not demonstrated this quality.19–21,36 Since fluoroquinolones are broadly available and used to treat many infectious diseases, concern has arisen regarding the development of drug resistance in patients with undiagnosed TB who are being treated with fluoroquinolones hence leading to diagnostic delay and resistance.29 Fluoroquinolone resistance is seen in patients previously treated with fluoroquinolones. Should fluoroquinolone resistance be, present bedaquiline should be considered as a substitute.37
Linezolid showed tuberculostatic activity in early off-label trials in combination regimens suggesting that the drug was active38 and evidence for this was brought by a prospective, randomised phase II clinical trial in patients with XDR-TB.39 Linezolid was effective in achieving culture conversion. However, 82% of patients developed significant adverse events, namely myelosuppression, peripheral neuropathy and optic neuropathy. Patients receiving 300mg per day had fewer adverse events than those who received 600mg per day throughout the study but may lead to acquired resistance. The swift addition of linezolid at a dose of 600mg per day to background treatment regimens improved the time to sputum culture conversion on solid medium, as compared with the delayed addition of linezolid; 34 out of the 39 patients (87%) had confirmed culture conversion within the six months of treatment.40,41
The benefit of the leprosy drug clofazimine, in sterilising MDR tuberculosis was suggested by several studies and trials31,42–44 and supported in a randomised controlled trial45 and a meta-analysis,46 that reported an overall pooled success rate of 61% (95% CI 52.79–71.12%) of regimens that included clofazimine, however studies included were heterogeneous. The pharmacokinetic characteristics of clofazimine as: its tissue distribution capacity, intracellular activity, and its prolonged half-life make this drug important for the design of second line regimens.47 Concerns regarding skin discoloration (possible stigmatisation and patient refusal), increased QT, cross-resistance with bedaquiline and pharmacokinetic drug–drug interactions may hinder its advance its use for drug-susceptible tuberculosis.
Carbapenems appear to have a role in MDR tuberculosis regimens, based on in vitro activity, case reports and recently promising results from a phase 2b randomised control early bactericidal activity trial. Meropenem, imipenem and ertapenem have been used with some success, in conjunction with other drugs, to treat patients with MDR-TB and XDR-TB.48–55 Meropenem plus clavulanate combination renders the Mycobacterial class A ß-lactamase, BlaC ineffective by clavulanate allowing for Mycobacterium tuberculosis to become susceptible to meropenem and amoxicillin. However, although the pharmacokinetic properties of meropenem (intravenous formulation and three times daily administration) preclude its general use for TB treatment, ertapenem a once daily carbapenem that can also be administered via intramuscular route, as well as intravenously, may be a valid solution.56 Attempts at using oral penem agents like faropenem, unfortunately, led to disappointing results in a recent early bactericidal study.55 A review of six drugs with antimicrobial activity against MTB (phenothiazine, metronidazole, doxycycline, disulfiram, tigecycline and co-trimoxazole), the sulfonamides appeared promising; they have been proposed as anti-tuberculosis drugs based on in vitro susceptibility, but no prospective trials have yet been done. Several studies of co-trimoxazole prophylaxis in HIV-infected people in Africa reported no effect on tuberculosis incidence.57–59 However, against actively replicating MTB, treatment dose co-trimoxazole seems to be active, its consistent pharmacokinetic profile, easy penetration into tissue and safety profile are advantages.60 A recent in vitro study demonstrated that mefloquine showed promising activity against TB and MDR-TB strains. Mefloquine is a drug with an excellent tissue penetration and a long half-life, well-tolerated by some, warrants further study.61 Clarithromycin previously a class 5 drug was removed from the WHO guidelines; however, a recent study suggests the drug is well tolerated and potentially has immunomodulatory effects that have not been explored.62
In summary, the evidence supporting repurposed drugs is growing even though most of the evidence is from observational studies, the fluoroquinolones and linezolid have proved to be effective and improve outcomes. Early bactericidal studies can demonstrate the antimycobactericidal efficacy of a single agent, however, these studies are less capable of demonstrating sterilising power against persisters.
The new compounds, bedaquiline, delamanid, pretomanid and related pipelineThe current tuberculosis antimicrobial drug pipeline shows eight drugs in phase 2–3 trials. Two new drugs (bedaquiline and delamanid) are in confirmatory phase 3 trials, having received accelerated approvals for MDR tuberculosis based on phase 2 data in 2012, and 2014, respectively. However, of the six remaining drugs, only two (sutezolid [an oxazolidinone] and pretomanid [PA-824; a nitroimidazole]) are new compounds. There are no current trials in progress for sutezolid, and hepatic safety concerns emerged during the largest trial of pretomanid. Ongoing studies of rifamycins (rifapentine [a long-acting but highly protein-bound rifamycin] and rifampicin) and fluoroquinolones (levofloxacin and moxifloxacin) seek mainly to optimise or define their roles in drug-susceptible tuberculosis. The WHO has recently produced guidelines to drug companies and research groups to target efforts in developing new drugs.63
The FDA and European medicines agency both gave conditional approval to both bedaquiline and delamanid to be used individually as a component of an optimised background regimen for pulmonary MDR-TB up to 24 weeks, they are not recommended for extra-pulmonary TB and efficacy in meningitis is currently lacking.64 WHO recently recommended bedaquiline or delamanid be considered as an additional drug for patients with drug-resistant tuberculosis where a regimen comprising four active drugs (not including pyrazinamide) cannot be made due to resistance or where intolerances or allergies exist. With appropriate criteria being met and informed consent. Reports suggesting the efficacy and safety of delamanid in children have recently been made available.51,65 WHO has released an interim recommendation allowing for the use of delamanid over the age six years as PK data was made available.66
Bedaquiline conversely is not recommended for use in children, but off-label use of Bedaquiline in children and adolescents (range age 10–7 years) with limited treatment options have been reported.67 Both bedaquiline and delamanid are not currently recommended in pregnancy. Both delamanid and bedaquiline may have to be used for more than 24 weeks in many pre-XDR and XDR-TB patients with few options,68 as removing these drugs may then compromise therapy.
Bedaquiline and delamanid have been co-administered as part of salvage regimens with satisfactory results.69–74 A recent WHO best-practice statement on the off-label co-administration of bedaquiline and delamanid while not recommending the combination has been produced to give guidance to support physicians in their decisions using these two drugs75, moreover the document discusses the importance and the responsibility of physicians to provide close patient monitoring for response to treatment and active drug safety monitoring and management (aDSM) as well as collection of detailed data.76 Two formalised clinical trials assessing the safety and efficacy of the combination have recently started enrolling: The U.S. National Institutes of Health's AIDS Clinical Trials Group protocol ACTG5343, which will evaluate the safety, tolerability, and pharmacokinetics of bedaquiline and delamanid alone and in combination; and the endTB trial, a randomised, controlled, open-label, Phase III trial evaluating the efficacy of several regimens for treatment of MDR-TB, including one combining bedaquiline and delamanid. Results of these trials, however, are only expected in three to five years.77,78
As data supporting the safety and efficacy of bedaquiline and delamanid accrue, the goal of research must be to define the role that they will play in future drug regimens. A large retrospective study was recently completed reporting safety and tolerability of bedaquiline in 428 patients, bedaquiline was interrupted due to adverse events in 5.8% of cases, 71.3% achieved success (62.4% cured; 8.9% completed treatment), 13.4% died, 7.3% defaulted and 7.7% failed, bedaquiline-containing regimens achieved high conversion and success rates under different non-experimental conditions.79
On the other hand, preliminary results of delamanid containing regimens under compassionate use in resource limited settings for MDR/XDR Tb patients have demonstrated culture conversion rates at 6 months 80%, and safety since QT prolongation (QTc>500ms) was present in only 3.8% of patients.80 Furthermore, in vitro and animal studies have demonstrated its activity against not only growing but also on dormant bacilli, so it is even proposed to be useful for the treatment of latent tuberculosis.
A regimen comprising the related compound, pretomanid another nitroimidazole drug, along with pyrazinamide and moxifloxacin, has been shown in phase II trials to possess early bactericidal activity against drug-susceptible tuberculosis at a level comparable to standard four-drug therapy81; the combination has also been shown to have sufficient bactericidal activity against drug-resistant tuberculosis.82 Phase III trials are required to assess this in more detail.
Current treatment courses for drug-resistant tuberculosis are lengthy; although the WHO now supports the use of the nine-month “Bangladesh” regimen,32 many patients do not meet the eligibility criteria and will still receive therapy of 18 months’ duration or more.31,83,84 Ideal treatment courses would be of dramatically shorter length than these current mainstream drug-resistant tuberculosis regimens. The ongoing STREAM trial aims to compare two new regimens both with standard therapy and a regimen closely modelled on the “Bangladesh” regimen.85 One trial arm includes bedaquiline in place of kanamycin, as part of an oral-only regimen; another arm adds bedaquiline in the context of an intensive regimen with a shortened treatment length of 28 weeks. An effective oral-only regimen has the potential to enormously simplify and render drug-resistant tuberculosis treatment more affordable, as would a regimen of shorter length – the results of this trial is awaited with great anticipation by the TB community. However, the current trend for trials to focus on a single novel agent neglects the benefits that may be gained from the incorporation of multiple new drugs into regimens. Very recently, preliminary data from a phase III trial (NIX-TB) of the all-oral regimen of bedaquiline, pretomanid, and linezolid (BPaL) was presented at the 47th Union conference.86 BPaL was shown to treat XDR-TB in 6 months. The interim results for the first 15 participants enrolled in the study, of whom 7 were HIV+, revealed that 12 of them completed six months of therapy. The majority was culture negative by week 8. As of the end of 2016, 30 patients had completed six months of treatment, and all had sputum converted. To date, there have been no relapses. However, the study is small and still ongoing. Four patients have died, however, a substantially lower mortality than ever reported before for XDR-TB.87 The results of this trial are possibly a game changer for the treatment of drug-resistant TB. The BPaL regimen goes so far as to alert us that now that we have two new drugs, we are close to obtaining a universal drug regimen capable of treating both susceptible and drug-resistant TB.
Despite the growing evidence base for bedaquiline and delamanid and their status as licensed drugs in various territories, they are yet to find their way into routine clinical practice in many countries. This is in part due to the incomplete nature of the evidence base, but also due to their current high cost and prescribing restrictions. A six-month course of bedaquiline in the United Kingdom currently costs nearly £20,000 and requires the approval of several independent specialist physicians on an advisory board and a central funding committee. Proponents of this system can reasonably argue that it protects the health system from an unnecessary financial burden, and perhaps, more importantly, protects bedaquiline from inappropriate use and thus the emergence of drug resistance88; detractors counter that there is little advantage in the existence of effective novel agents if they cannot rapidly be deployed when clinically indicated. It is clear that whatever novel treatment regimens emerge from future clinical trials, political and financial frameworks will need to be established to support their implementation, both in resource-limited and rich settings. Although the drugs above are the most advanced of the novel agents, there are a variety of other compounds undergoing earlier stage clinical trials. Sutezolid, an oxazolidinone, appears to cause fewer adverse events than its first-in-class cousin linezolid, and may, therefore, be better tolerated for long courses of treatment; AZD5847 is another candidate in this class in Phase II trials (NCT01516203). Q203, a candidate compound that targets the mycobacterial respiratory chain, is currently undergoing a Phase I trial (NCT02530710). SQ109, a structural derivative of ethambutol, targeting the mycobacterial transport protein MmpL3 amongst other aspects of bacterial machinery, is currently undergoing phase II trials (NCT01785186).89 For the first time in decades, the prospect of a much broader TB drug armamentarium seems feasible, and there is cause for cautious optimism. However, it is vital that these new agents are introduced in a manner that will protect them from the early emergence of resistance – already detected in bedaquiline and delamanid otherwise they will be rendered ineffectual before their full potential can be realised.88,90
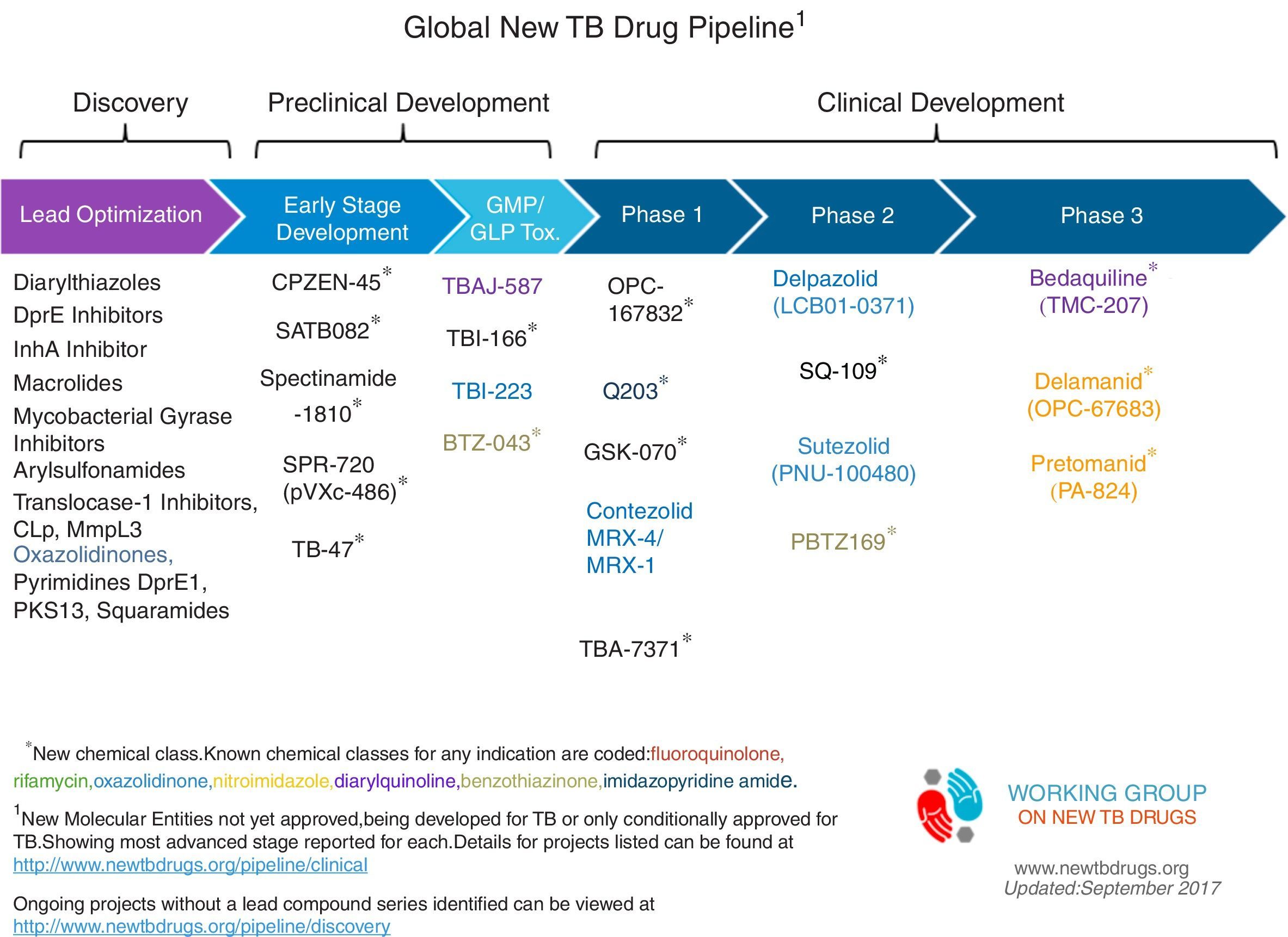
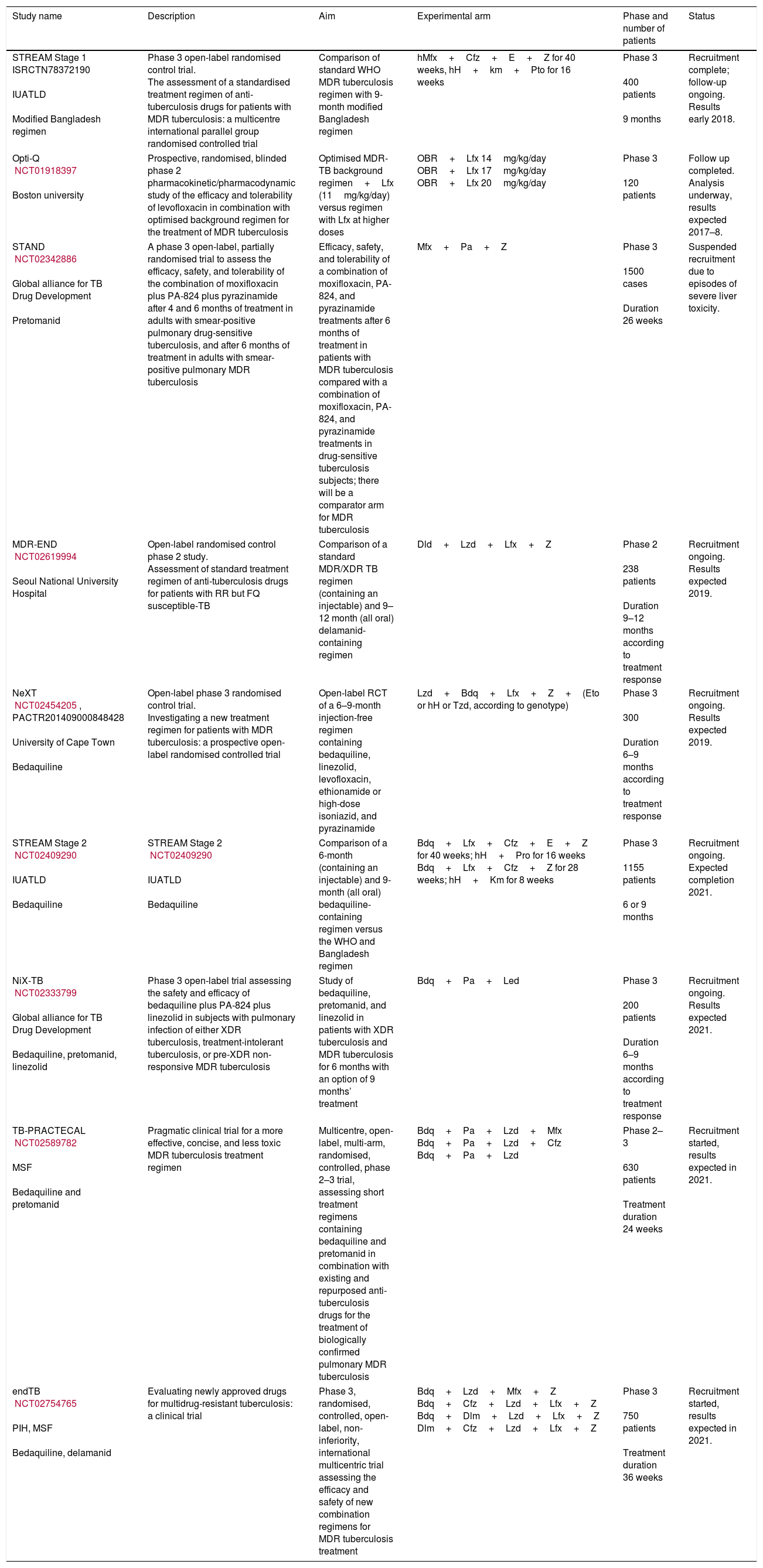
The problems of obtaining the evidence to support changes in practice through clinical trials in drug-resistant TB are multiple, but the key is the lack of an adequate gold standard against which to test new treatments and problematic outcome measures.91 A full list of current drug-resistant trials produced by Resist-TB can be seen in Fig. 1 and Table 2.
Global TB drug pipeline, reproduced with permission. https://www.newtbdrugs.org/pipeline/clinical.
Selected Clinical Phase 2 and 3 TB drug trials.
| Study name | Description | Aim | Experimental arm | Phase and number of patients | Status |
|---|---|---|---|---|---|
| STREAM Stage 1 ISRCTN78372190 IUATLD Modified Bangladesh regimen | Phase 3 open-label randomised control trial. The assessment of a standardised treatment regimen of anti-tuberculosis drugs for patients with MDR tuberculosis: a multicentre international parallel group randomised controlled trial | Comparison of standard WHO MDR tuberculosis regimen with 9-month modified Bangladesh regimen | hMfx+Cfz+E+Z for 40 weeks, hH+km+Pto for 16 weeks | Phase 3 400 patients 9 months | Recruitment complete; follow-up ongoing. Results early 2018. |
| Opti-Q NCT01918397 Boston university | Prospective, randomised, blinded phase 2 pharmacokinetic/pharmacodynamic study of the efficacy and tolerability of levofloxacin in combination with optimised background regimen for the treatment of MDR tuberculosis | Optimised MDR-TB background regimen+Lfx (11mg/kg/day) versus regimen with Lfx at higher doses | OBR+Lfx 14mg/kg/day OBR+Lfx 17mg/kg/day OBR+Lfx 20mg/kg/day | Phase 3 120 patients | Follow up completed. Analysis underway, results expected 2017–8. |
| STAND NCT02342886 Global alliance for TB Drug Development Pretomanid | A phase 3 open-label, partially randomised trial to assess the efficacy, safety, and tolerability of the combination of moxifloxacin plus PA-824 plus pyrazinamide after 4 and 6 months of treatment in adults with smear-positive pulmonary drug-sensitive tuberculosis, and after 6 months of treatment in adults with smear-positive pulmonary MDR tuberculosis | Efficacy, safety, and tolerability of a combination of moxifloxacin, PA-824, and pyrazinamide treatments after 6 months of treatment in patients with MDR tuberculosis compared with a combination of moxifloxacin, PA-824, and pyrazinamide treatments in drug-sensitive tuberculosis subjects; there will be a comparator arm for MDR tuberculosis | Mfx+Pa+Z | Phase 3 1500 cases Duration 26 weeks | Suspended recruitment due to episodes of severe liver toxicity. |
| MDR-END NCT02619994 Seoul National University Hospital | Open-label randomised control phase 2 study. Assessment of standard treatment regimen of anti-tuberculosis drugs for patients with RR but FQ susceptible-TB | Comparison of a standard MDR/XDR TB regimen (containing an injectable) and 9–12 month (all oral) delamanid-containing regimen | Dld+Lzd+Lfx+Z | Phase 2 238 patients Duration 9–12 months according to treatment response | Recruitment ongoing. Results expected 2019. |
| NeXT NCT02454205, PACTR201409000848428 University of Cape Town Bedaquiline | Open-label phase 3 randomised control trial. Investigating a new treatment regimen for patients with MDR tuberculosis: a prospective open-label randomised controlled trial | Open-label RCT of a 6–9-month injection-free regimen containing bedaquiline, linezolid, levofloxacin, ethionamide or high-dose isoniazid, and pyrazinamide | Lzd+Bdq+Lfx+Z+(Eto or hH or Tzd, according to genotype) | Phase 3 300 Duration 6–9 months according to treatment response | Recruitment ongoing. Results expected 2019. |
| STREAM Stage 2 NCT02409290 IUATLD Bedaquiline | STREAM Stage 2 NCT02409290 IUATLD Bedaquiline | Comparison of a 6-month (containing an injectable) and 9-month (all oral) bedaquiline-containing regimen versus the WHO and Bangladesh regimen | Bdq+Lfx+Cfz+E+Z for 40 weeks; hH+Pro for 16 weeks Bdq+Lfx+Cfz+Z for 28 weeks; hH+Km for 8 weeks | Phase 3 1155 patients 6 or 9 months | Recruitment ongoing. Expected completion 2021. |
| NiX-TB NCT02333799 Global alliance for TB Drug Development Bedaquiline, pretomanid, linezolid | Phase 3 open-label trial assessing the safety and efficacy of bedaquiline plus PA-824 plus linezolid in subjects with pulmonary infection of either XDR tuberculosis, treatment-intolerant tuberculosis, or pre-XDR non-responsive MDR tuberculosis | Study of bedaquiline, pretomanid, and linezolid in patients with XDR tuberculosis and MDR tuberculosis for 6 months with an option of 9 months’ treatment | Bdq+Pa+Led | Phase 3 200 patients Duration 6–9 months according to treatment response | Recruitment ongoing. Results expected 2021. |
| TB-PRACTECAL NCT02589782 MSF Bedaquiline and pretomanid | Pragmatic clinical trial for a more effective, concise, and less toxic MDR tuberculosis treatment regimen | Multicentre, open-label, multi-arm, randomised, controlled, phase 2–3 trial, assessing short treatment regimens containing bedaquiline and pretomanid in combination with existing and repurposed anti-tuberculosis drugs for the treatment of biologically confirmed pulmonary MDR tuberculosis | Bdq+Pa+Lzd+Mfx Bdq+Pa+Lzd+Cfz Bdq+Pa+Lzd | Phase 2–3 630 patients Treatment duration 24 weeks | Recruitment started, results expected in 2021. |
| endTB NCT02754765 PIH, MSF Bedaquiline, delamanid | Evaluating newly approved drugs for multidrug-resistant tuberculosis: a clinical trial | Phase 3, randomised, controlled, open-label, non-inferiority, international multicentric trial assessing the efficacy and safety of new combination regimens for MDR tuberculosis treatment | Bdq+Lzd+Mfx+Z Bdq+Cfz+Lzd+Lfx+Z Bdq+Dlm+Lzd+Lfx+Z Dlm+Cfz+Lzd+Lfx+Z | Phase 3 750 patients Treatment duration 36 weeks | Recruitment started, results expected in 2021. |
As the prevalence of drug resistance rises, the question of how to manage MDR-TB contacts and those with possible latent MDR-TB will become increasingly important. TB-Champ is a phase III multi-centre, community-based, cluster randomised controlled trial of levofloxacin versus placebo for the treatment of child and adolescent household contacts of MDR-TB which started recruiting in 2016. Similarly, PHOE-Nix and V-QUIN include both adults and children and are examining the efficacy of bedaquiline and levofloxacin respectively versus placebo.92 Of importance to low-incidence countries, amongst which transmission rates are low, is the increasing prevalence of extra-pulmonary tuberculosis and its associated individual morbidity and mortality.93,94 While culture conversion remains the bacteriological outcome measure, trials amongst patients with extra-pulmonary tuberculosis remain a challenge. Finally, we must find affordable and effective regimens that work in the real world which include children, pregnant women and those with viral co-infections amongst others.
ConclusionMDR-TB is a significant challenge for the control of TB in many parts of the world and a threat to TB elimination. The origin of this problem has been the sub-optimal management, individual or programmatic, of patients with susceptible TB. Inadequate management of cases can be the origin of 50% of the new RR/MDR-TB cases. The other 50% is due to active transmission of RR/MDR-TB strains in the community or healthcare settings. Therefore, to control this epidemic, we will need to improve the management of the susceptible TB cases as well as find and cure most of the RR/MDR- TB cases, to whom best treatment options must be assured.
Fortunately, after almost four decades with practically the same diagnostic tools and armamentarium, there have been significant advances in this field, with a focus on the new global drug-resistant TB epidemic. Drug resistant TB treatment has evolved considerably over the last years, the new shorter MDR-TB regimens and increasing availability of new or repurposed drugs like bedaquiline, delamanid, clofazimine and linezolid i tis envisaged that more patients will be able to be treated and more will survive. If we are careful, we will not repeat previous errors with the new drugs and the dream of developing a universal new regimen with these new drugs for all TB patients, susceptible and resistant to all the old drugs may become a reality.
Conflicts of interestThe authors have no conflicts of interest to declare.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The reason for having seven authors instead of six was the need to complete a thorough manuscript within the given deadlines.