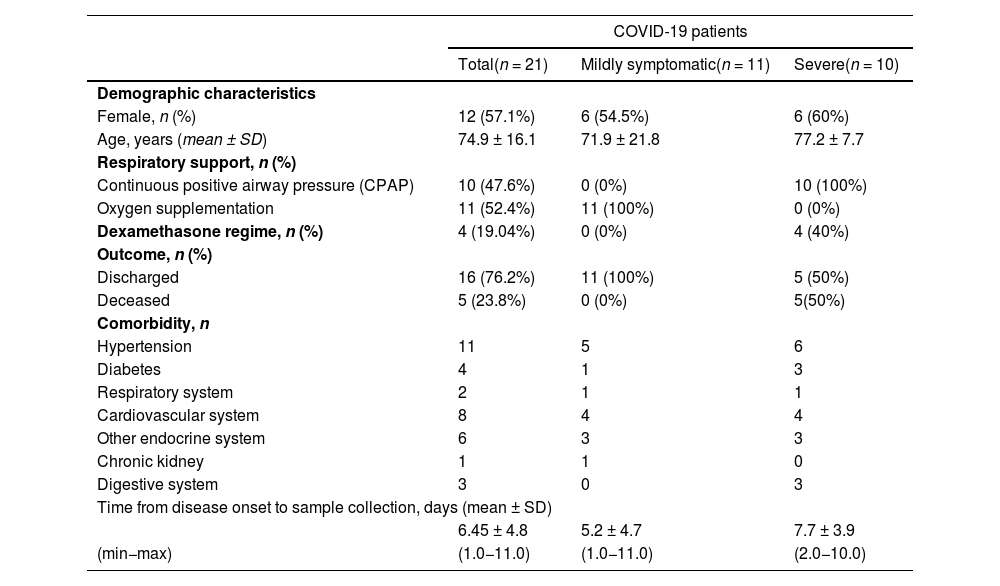
Peptidomics is an innovative technique that allows the identification of endogenous or foreign peptides associated with pathogenic organisms and infective agents, potentially exploited to better understand COVID-19 pathophysiology. To date, the plasma peptidome of COVID-19 patients has been poorly characterized due to the challenging sample preparation requirements, peptide stability, and analytical issues including the wide range of peptide polarity and concentration.1 For these reasons, in a recently published work, we applied for the first time an untargeted mass spectrometry-based peptidomic approach to plasma samples from patients infected by SARS-CoV-2 virus.2 Since we already discussed the mainly observed peptidomic differences occurring between COVID-19 positive patients and negative controls, here we focus on the comparison of the peptidome of plasma samples from mildly symptomatic SARS-CoV-2 infected (n = 11) patients and critical ones (n = 10). Mildly symptomatic subjects required only low-flow oxygen supplementation, whereas critical patients were those admitted to the semi-intensive respiratory unit care with respiratory failure requiring at least non-invasive ventilation (continuous positive airway pressure, CPAP). The clinical characteristics and comorbidities of the patients are reported in Table 1. Other data of patients have been published elsewhere.2 The Institutional Review Board (Comitato Etico Interaziendale Novara) approved this study (no. RQ06320/25 March 2020) including all permissions taken from the patients.
Clinical data of the patients.
Sample collection carried out at admission to hospital. Data are presented as number and percentage for dichotomous values or mean ± SD for continuous values.
QAE Sephadex A-25 strong anion exchange particles (Sigma–Aldrich St. Louis, MO, USA) were used to fractionate 200 µL of citrate plasma samples. After that, peptides were desorbed from highly abundant proteins by heating samples to 60 °C for 15 min.3 The circulating peptidome was investigated using the micro-LC Eksigent Technologies (Eksigent, Dublin, USA) system linked to a TripleTOF 5600+ mass spectrometer (AB Sciex, Concord, Canada). The plasma peptidome was then identified using a database search.
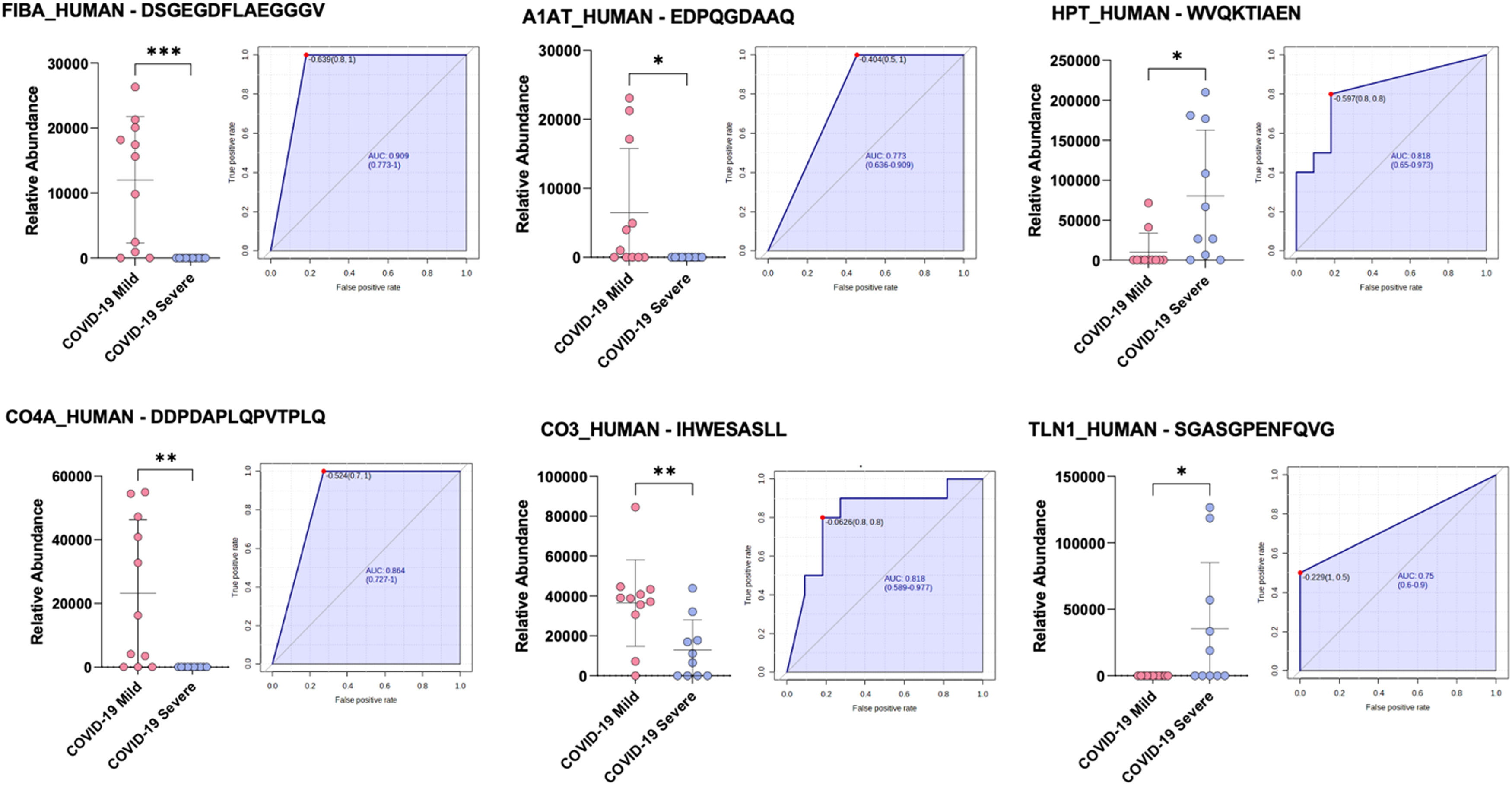
The subsequent statistical analysis based on a t-test and the ratio of the abundances of quantified peptides within each group (p-value ≤ 0.05, fold change ≥ 1.3), revealed the presence of only 9 regulated peptides in plasma samples from mildly symptomatic COVID-19 vs critical patients, 5 of which were overexpressed in the mild group and 4 in those experiencing the severe disease.
More specifically, mild symptomatic patients presented an overexpression of two peptides belonging to Isoform 2 of Fibrinogen alpha chain (FIBA_HUMAN), DSGEGDFLAEGGGV and DEAGSEADHEGTHST, one peptide deriving from Isoform 2 of Complement C4-A (CO4A_HUMAN), DDPDAPLQPVTPLQ, one Alpha-1-antitrypsin (A1AT_HUMAN) related peptide, EDPQGDAAQ, and a Complement C3 (CO3_HUMAN) peptide, IHWESASLL.
Conversely, the critical group was characterized by the up modulation of two peptides related to Isoform 2 of Haptoglobin (HPT_HUMAN), WVQKTIAEN and VDSGNDVTDIADD, one peptide from Transthyretin (TTHY_HUMAN), LSPYSYSTTAVVTNPKE, and one peptide belonging to Talin (TLN1_HUMAN), SGASGPENFQVG. In addition, these 9 peptides were not expressed by all the patients. In particular, the following peptides DSGEGDFLAEGGGV and DEAGSEADHEGTHST both from FIBA, DDPDAPLQPVTPLQ (from A1AT1) and EDPQGDAAQ (from CO4A) were not present in severe patients, while SGASGPENFQVG (from TLN1) was not expressed in mild patients.
We also investigated, using boxplots and ROC curves, the possibility of modulated peptides to be indicative of the clinical different course of SARS-CoV-2 infection, and we observed that 6 of them performed optimally in diagnostic tests (Fig. 1): FIBA_HUMAN-DSGEGDFLAEGGGV (AUC = 0.909), A1AT_HUMAN-EDPQGDAAQ (AUC = 0.773), CO4A_HUMAN-DDPDAPLQPVTPLQ (AUC = 0.864), CO3_HUMAN-IHWESASLL (AUC = 0.818), HPT_HUMAN-WVQKTIAEN (AUC = 0.818), and TLN1_HUMAN-SGASGPENFQVG (AUC = 0.75).
Boxplots and ROC curves for the best potential biomarkers identified with peptidomic analysis (pink dots: COVID-19 mild, purple dots: COVID-19 severe). ***p <0.001; ** p < 0.01; * p < 0.05. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Our data show that the main regulated peptides in both mildly symptomatic and severe COVID-19 patients derive from inflammatory, immune-response and coagulation proteins and that specific peptide's overexpression suggest a correlation with severity of disease. In particular we found important differences among overexpressed peptides in mild symptomatic and severe patients. For example, we observed a stronger upregulation of transthyretin, of haptoglobin peptides and of a peptide derived from the cytoskeletal protein Talin, and conversely, a downregulation of fibrinogen, complement, and alpha-1-antitrypsin peptides in severe versus mild cases. Especially in the critical group the meaning of the up-regulated peptides (HPT_HUMAN, TTHY_HUMAN, TLN1_HUMAN) is not known and still needs to be addressed. Interestingly, the quantification of parental proteins show no modulation in mild vs severe COVID 19 patients4 suggesting that the observed differences are due to modulation of peptides metabolism more than mirroring the levels of the full protein.
HPT_HUMAN peptide, for example, which is involved in neutralizing free heme directly linked to the infection's increased hemolysis seems to be overwhelmed in severe SARS-CoV-2 patients, therefore, leading us to postulate that infected subjects show high levels of the related protein unable to efficiently neutralize free heme, thus suggesting a modified degradation pattern.5
Transthyretin (TTHY_HUMAN) is an acute phase-reactant acting as a hormone transporter whose levels negatively correlate with inflammation, it is known to be a neuroprotective and oxidative-stress-suppressing factor and low concentrations of TTHY are indicative of a systemic inflammatory state.6 In severe COVID-19 patients we observed an overexpression of this peptide that may be related to high-dose steroid treatment (glucocorticoids increase transthyretin plasmatic level)7 or to an altered degradation pathway and could be an attempt of homeostasis correction of a very de-regulated background.
Talin-1 is a cytoskeletal protein central for the regulation of cell-matrix adhesion and its depletion is responsible of severely affected focal adhesion assembly. High plasma sTalin-1 levels in patients with coronary artery disease (CAD) were found to be associated with severity of CAD, suggesting a role of sTalin-1 in the progression of coronary atherosclerosis,8 hence an overexpression of this peptide may have a potential role in cardiovascular consequences of severe COVID-19 infection.
To date this is the only study investigating how SARS-CoV-2 infection affects circulating peptides, further research would allow the identification of more specific pathways and peptides associated with the development of the disease.
Taken the limitations of a small sample and the preliminary value of our findings, it is interesting to observe that we found some modulated peptides to be good diagnostic tools in predicting different outcomes of COVID-19 infection.
In conclusion, the changes observed in circulating peptidome are the result of a complex balance between target abundance, protease activity, and clearance rate, all of which might be modulated by COVID-19 infection.