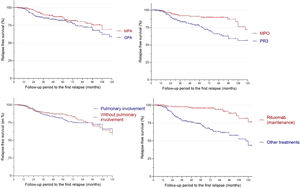
In a recent study the French Vasculitis Study Group identified overall predictive factors for AAV relapse, including PR3-ANCA, age under 75 years, and eGFR greater than 30 ml/min/1.73m2.1 We aim to examine factors that specifically contribute to pulmonary relapse in patients with microscopic polyangiitis (MPA) and granulomatosis with polyangiitis (GPA). This study included adult patients with MPA or GPA who were followed at Toulouse University Hospital (France) between 2004 and 2019. The local ethics committee approved the study and written consent was waived in accordance with French law on retrospective observational studies. Diagnosis of AAV was based on clinical and biological criteria of vasculitis and histological findings.2 Patients were defined as relapsed when symptoms of active vasculitis returned, and other causes were excluded. Relapse-free survival was calculated from the diagnosis date to relapse date or the last follow-up date for patients who did not relapse. The Kaplan-Meier estimator was used to generate relapse-free survival probability curves and they were compared using log-rank tests. The Cox model was used to estimate relative risks of relapse based on potential prognostic variables. Variables associated with relapse with a statistical threshold of less than 20% were included in the models, except for variables for which more than 50% of the data was missing. Stata software version 14.2 (StataCorp) was used for analyses. A total of 274 patients with AAV were included in this study, of which 147 (53.6%) were male and 133 (48.5%) had GPA. PR3-ANCA positivity was observed in 81% GPA patients, while 76.6% of MPA patients were MPO-ANCA positive. The most commonly used treatments for induction-remission were intravenous cyclophosphamide (55%) and rituximab (39%) and for maintenance-remission rituximab (38%) and azathioprine (32%). No significant difference in therapeutic approaches was observed between MPA and GPA patients. The mean follow-up period was 70 months (SD: 53.0) and the median duration from diagnosis to the first relapse was 48 months (SD: 37.5). We observed 86 relapses (31.4%) and 33 with pulmonary relapse (12%). AAV patients' cumulative probability of relapse at 1-year, 3-year, and 5-year intervals was 2%, 17%, and 21%, respectively. There was no significant difference in relapse rate based on the patients’ AAV diagnosis (p=0.23) or whether they had pulmonary involvement (p=0.66) or not (Fig. 1). However, relapse occurred more frequently in anti-PR3 patients (p=0.0007) and those who had not received rituximab as maintenance therapy (p<0.0001) (Fig. 1). The multivariate analysis excluded 24 patients (8.6%) due to lack of follow-up data. Risk factors associated with pulmonary relapse, as identified, included initial pulmonary involvement (HR 9.6; 95% CI [1.2; 74.6]; p=0.03), cardiac involvement (HR 6.4; 95% CI [1.7; 24.3]; p=0.006), mechanical ventilation (HR 21.6; 95% CI [1.9; 247.5]; p=0.014), and the presence of cavitary lung lesions (HR 5.2; 95% CI [1.7; 15.8]; p=0.004). Using rituximab as an induction-remission therapy was a protective factor, with a four-fold lower risk of pulmonary relapse (HR 0.23; 95% CI [0.06; 0.86]; p=0.03). Death rate was 10% without significant difference between MPA and GPA patients (p=0.20). Achieving remission in GPA or MPA patients is often challenging. One-third of patients experienced an AAV relapse in 4 years in our study consistent with long-term follow-up studies.1,3 Previous reports have conflicting data on the association between pulmonary involvement and risk of relapse.3,4 We found that pulmonary involvement is not associated with an increased risk of overall relapse but it is associated with a nearly 10-fold higher risk of pulmonary relapse. In multivariate analysis, a more severe diffuse alveolar hemorrhage (DAH) requiring mechanical ventilation and blood transfusions (indicative of active vasculitis disease) and the presence of cavitated nodules (indicative of active granulomatous disease) were specific predictive factors for pulmonary relapse. Previous studies suggested that cardiac involvement was associated with an overall risk of relapse1,5 and our data are consistent with these studies (for pulmonary relapse). The underlying physiopathological mechanism remains unknown and caution is necessary due to the low rate of this condition. In our study, using rituximab in maintenance therapy is associated with reduced risk of relapse, consistent with results from randomized trials.6,7 Furthermore, in multivariate analysis, using rituximab in induction therapy was associated with improved respiratory outcomes and a four-fold lower risk of pulmonary relapse. We restricted ourselves to ANCA-positive vasculitis through a classification bias present. The retrospective and monocentric design of the study, coupled with the potential for missing data and referral biases, poses a significant challenge to establishing causal relationships. However, the large sample size can help to reduce the impact of bias and enhance the generalizability of the findings. Risk factors for pulmonary relapse include pulmonary involvement with severe DAH or cavitary nodules at disease onset and cardiac involvement. Identifying these risk factors early can enable tailored treatment strategies with rituximab and monitoring pulmonary relapse risk during follow-up.
The Impact Factor measures the average number of citations received in a particular year by papers published in the journal during the two preceding years.
© Clarivate Analytics, Journal Citation Reports 2025
SRJ is a prestige metric based on the idea that not all citations are the same. SJR uses a similar algorithm as the Google page rank; it provides a quantitative and qualitative measure of the journal's impact.
See moreSNIP measures contextual citation impact by wighting citations based on the total number of citations in a subject field.
See more