Lung involvement can occur in 10–80% of rheumatoid arthritis (RA) patients,1,2 mostly within the first 5 years of RA diagnosis.1 It includes interstitial lung disease (ILD), airways disease, pleural disease and nodules.1 Pulmonary hypertension and direct toxicity from RA therapy have also been described.1
Despite therapeutic advances,2 lung disease remains responsible for 10–20% of RA mortality.1
This observational, retrospective, multicenter study characterizes lung involvement in a nationwide cohort of RA patients, identifies factors associated with lung disease and describes treatments used in patients with RA-ILD. All RA patients aged ≥18 years at diagnosis prospectively followed in Rheumatic Diseases Portuguese Registry (Reuma.pt) were included from 2008 to February 2022.
Lung involvement diagnosis was based on high resolution computed tomography (CT) and/or histopathological data. The date of the exam was considered the date of lung disease diagnosis. Demographics and clinical data, including smoking habits, RA duration, rheumatoid factor (RF), anti-citrullinated peptide antibodies (ACPA), secondary Sjögren's syndrome (SS2) and subcutaneous rheumatoid nodules, were retrieved at last visit. Erosive disease was based on X-rays performed at any time. Current/previous disease modifying antirheumatic drugs (DMARDs) and antifibrotics were recorded. Missing information was filled in from hospital records, whenever possible.
Continuous variables were expressed as mean ± S.D. or median with interquartile range (IQR) and categorical variables as absolute values and frequencies. Groups were compared using qui-square test and independent samples t test or Mann–Whitney test, as appropriate. Statistically significantly different variables were included in a logistic regression analysis for identifying features independently associated with lung disease. A significance level of 5% was considered. SPSS version 28.0 (IBM Corp, Armonk, NY, USA) was used. The study was approved by the Coordinator and Scientific Board of Reuma.pt and by the Ethics Committee of Hospital Garcia de Orta. All data registered on Reuma.pt is subjected to an informed consent signed by the patient.
From 9415 RA patients registered in Reuma.pt, 7473 (79.4%) were female, with mean age of 62.3 ± 13.6 years and median disease duration of 12.4 [IQR 6.5–20.6] years at last visit.
Lung disease was documented in 298 (3.2%) patients. The median interval between joint and pulmonary symptoms was 5 [IQR 1–15] years. Twenty-one (7.8%; 28 missing data) patients had lung disease as first manifestation.
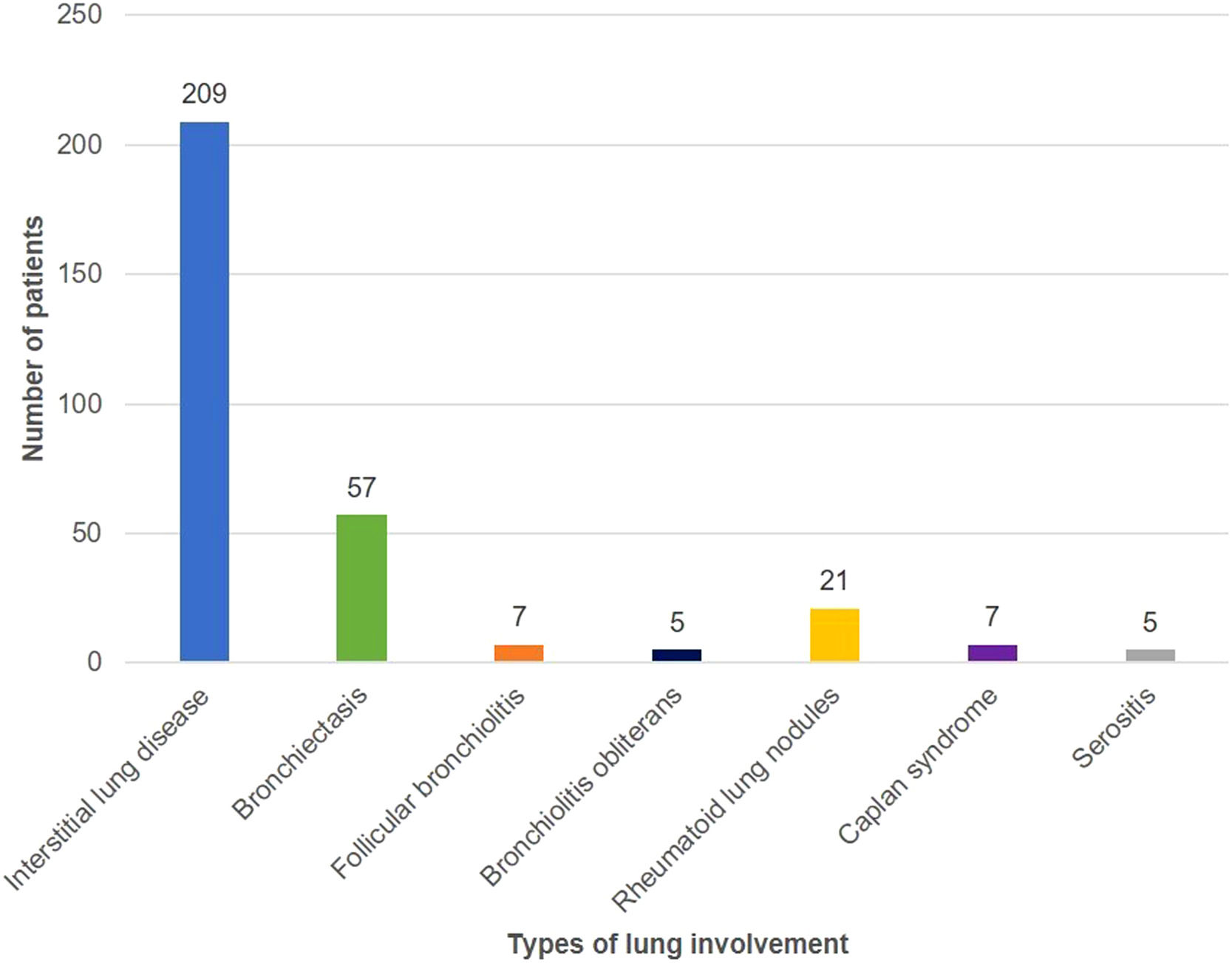
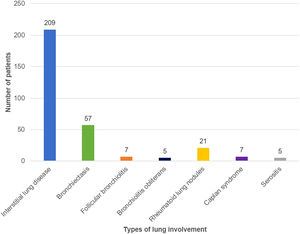
Fig. 1 shows the distribution of different types of lung involvement. Thirteen patients had more than one type of lung involvement. None of the patients with rheumatoid lung nodules had subcutaneous nodules. Two patients with CT showing peribronchial micronodules and a fluffy “tree-in-bud pattern” had lung biopsy, which documented follicular bronchiolitis.
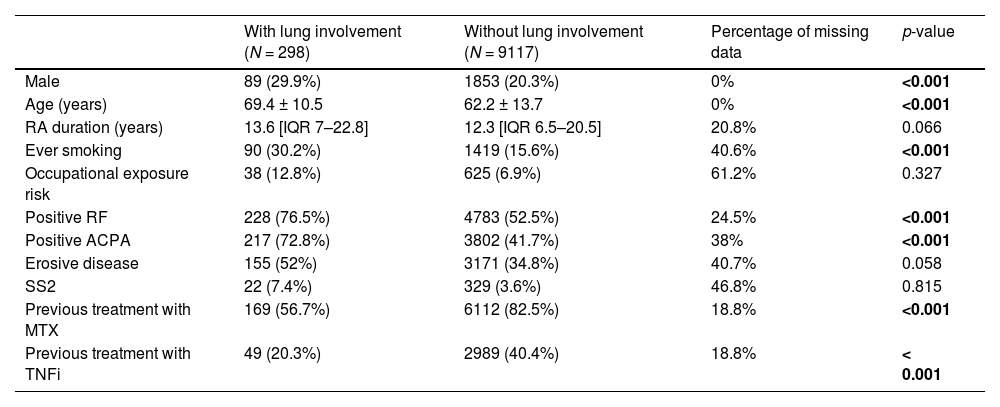
Table 1 shows clinical characteristics of patients with and without lung involvement.
Comparison of clinical characteristics between patients with and without lung involvement.
RA, rheumatoid arthritis; RF, rheumatoid factor; ACPA, anti-citrullinated peptide antibody; SS2, secondary Sjögren's syndrome; MTX, methotrexate; TNFi, tumor necrosis factor inhibitor.
Continuous variables are expressed as mean ± S.D. if they had a normal distribution, or median with interquartile range (IQR) if not normally distributed. Categorical variables are presented as absolute values (n) and frequencies (%).
In multivariate analysis, ever smoking (OR = 2.1; [95%CI:1.4–3.9], p < 0.001), positive ACPA (OR = 2.1; [95%CI:1.2–3.6], p = 0.002) and older age (OR = 1.05 per year; [95%CI:1.03–1.07], p < 0.001) were positively associated with lung disease, whereas previous treatment with methotrexate (MTX) (OR = 0.32; [95%CI:0.22–0.46], p < 0.001) and tumor necrosis factor inhibitors (TNFi) (OR = 0.48; [95%CI: 0.32–0.7], p < 0.001) had a negative association with RA-associated lung disease.
At lung disease diagnosis, 169 (56.7%) patients were taking MTX, 131 (44%) other conventional synthetic DMARDs, 49 (16.4%) TNFi, 13 (4.4%) tocilizumab, 9 (3%) rituximab, 2 (0.7%) abatacept and 2 (0.7%) Janus Kinase inhibitors. After lung disease diagnosis, 77 out of 169 patients (45.6%) were kept under MTX.
After ILD diagnosis, rituximab became the most prescribed biologic, in 62 (34.1%) patients, followed by tocilizumab (15 patients; 8.2%) and abatacept (7 patients; 3.8%). TNFi were used in 16 (8.8%) patients. Twelve RA-ILD patients received antifibrotics (6 nintedanib, 6 pirfenidone).
The proportion of RA patients with lung involvement in our cohort was lower than that reported in the literature,1 which might be explained by underreporting in Reuma.pt and because in most cases lung disease was only screened after respiratory symptoms developed. High-resolution CT is now considered the gold-standard for diagnosis. Lung biopsy is only performed when imaging features are inconclusive or when the etiology of lung disease is unclear.1,2
Besides, taking into consideration the pathogenic role of RA-related autoantibodies, smoking and occupational dust in RA-associated lung disease1,3 and the fact that articular disease activity seems to be higher in patients with RA-ILD,1 the lower smoking prevalence rates in Portugal compared to other European countries4 and the lower aggressiveness of RA in southern Europe than in other geographic areas5 can also contribute to the lower prevalence of RA-associated lung disease in our cohort. In 7.8% of RA patients, lung disease was the first disease manifestation, with recent data suggesting that the lung may be a potential mucosal site of generation of RA-related autoimmunity.3
RA-ILD was the most prevalent type of lung involvement (70.1% of the patients with lung disease), which is in line with published data.1
Patients with RA-associated lung disease had a higher frequency of smoking habits, positive RF and ACPA and erosive disease, consistent with the literature.1,3 However, 24 patients with lung disease were negative for RF and ACPA, with only 2 having smoking habits. This means that other factors may contribute to lung disease development.
Data on MTX and others DMARDs causing/worsening pre-existing ILD in RA patients is controversial,2,6 with recent data demonstrating that controlling systemic inflammation can delay/prevent RA-ILD development.3
Despite being a retrospective study, this constitutes an extensive characterization of a considerable number of RA patients with lung involvement and confirms the reproducibility of classic risk factors for lung disease7 in a national cohort. In the future, the identification of new risk factors and the validation of risk scores, could help identify patients at high risk of RA-associated lung disease that might benefit from a screening strategy including an HRCT at disease diagnosis, similar to what is already recommended for systemic sclerosis.8
The involvement of several centers explains why the number of 6 authors has been exceeded.