Biologics represent an opportunity for severe, uncontrolled, T2-high asthmatic patients. Despite their careful prescription, response can be incomplete and a switch to another agent can be proposed.
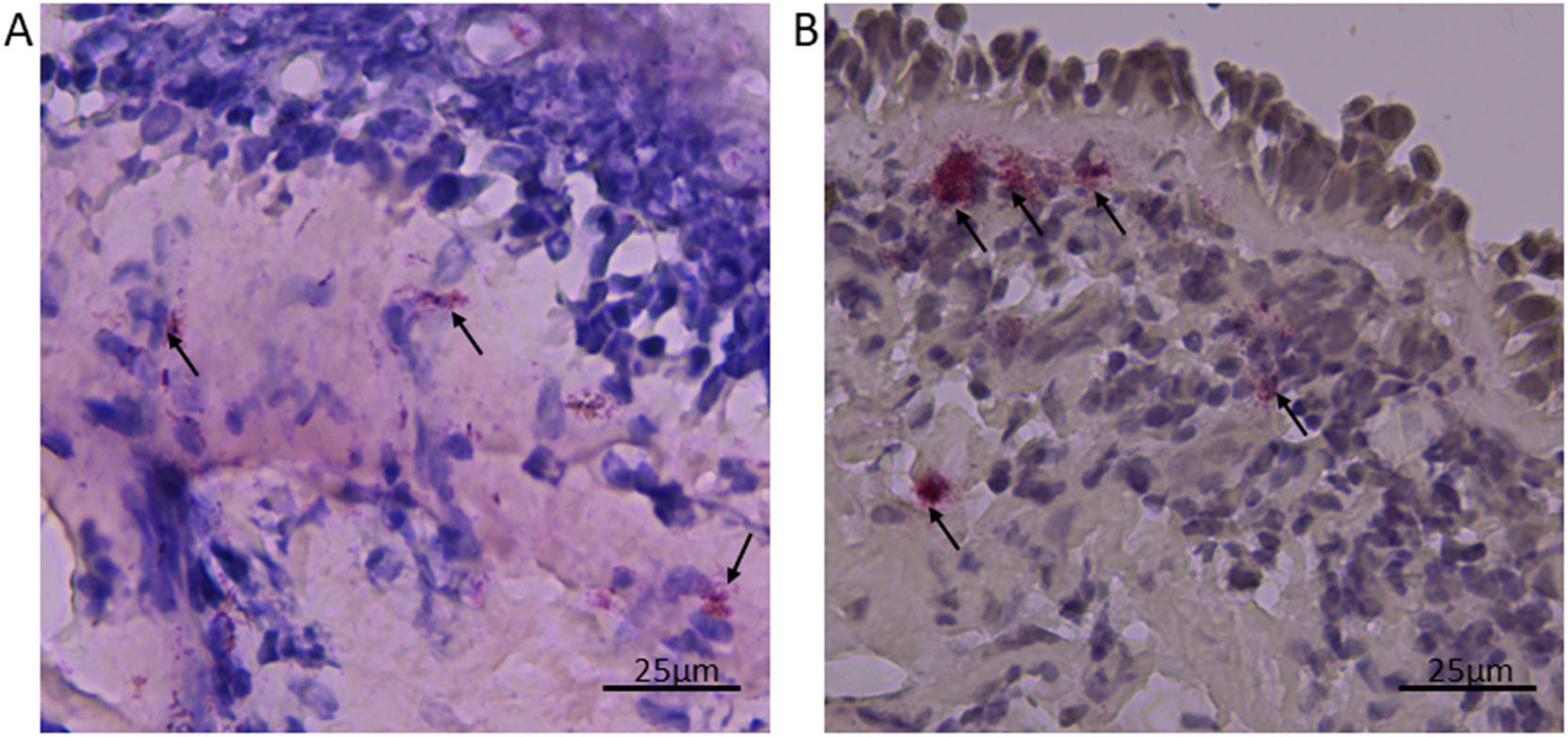
A 69-year-old, never-smoker woman was admitted in 2009 to the Severe Asthma Unit with uncontrolled asthma symptoms. She was sensitized to dust mites and suffered from nasal polyps (NPs) relapsing 6 times after surgery. Daily maintenance treatment consisted of high ICS dose + long-acting beta-agonists and prednisone 5 mg. She also needed 6 bursts of OCS/year for asthma exacerbations (AEs). Pulmonary function was severely compromised (FEV1 0.69 L, FEV1/FVC 32%), and the other parameters showed FENO 18 ppb, Peripheral Blood Eosinophils (PBE) 1060 cells/µL, total IgE 198 UI/mL, ACT 11/25 and Sinonasal Outcome Test (SNOT-22) 58. She underwent fiberoptic bronchoscopy (protocol 1759/2008-14871/2009) and bronchial biopsies were collected (Fig. 1).
At cell count, eosinophils were 23.58 cells/mm2 and neutrophils 108.49 cells/mm2, both above the normality thresholds.1 Other diagnoses were excluded. Asthma was classified as severe, late-onset, poorly controlled, frequently exacerbating with T2 phenotype2 and mixed inflammatory profile.
Anti-IgE Omalizumab was prescribed and, after 8 years of treatment, ACT and SNOT-22 improved, PBE decreased as well as AEs (3/year), but FEV1 unchanged. Daily ICS dose was reduced but still needing OCS. Therefore, Omalizumab was withdrawn due to an unsatisfying response.
In October 2017, after 3 months of washout, she started anti-IL5 Mepolizumab (1° switch) leading, after 3 years of treatment, to a good clinical and laboratory response with PBE falling to 130 cells/µL and further ICS dose reduction. Daily OCS was no longer needed and AEs were abolished. However, the patient still complained of bothering nasal symptoms due to recurrence of NPs. Mepolizumab was interrupted and, following 3 months of washout, anti-IL-4Rα Dupilumab was prescribed (2° switch).
After just 3 weeks of therapy the patient showed great amelioration in terms of ACT and SNOT-22 score, FEV1 and FENO values. As expected, PBE mildly increased. In March 2021, after 2 months, the patient complained of persistent tachycardia and PBE raised to 2380 cells/µL. Based on these side effects, Dupilumab was interrupted.
After 4 months of a wash-out, asthma was uncontrolled and exacerbated, leading to starting anti-IL5Rα Benralizumab (3° switch). After 6 months, clinical and functional parameters improved, AEs were abolished and PBE dropped to 0 cells/µL (Table 1). Facial RM showed a residual inflammatory tissue in osteomeatal complexes.
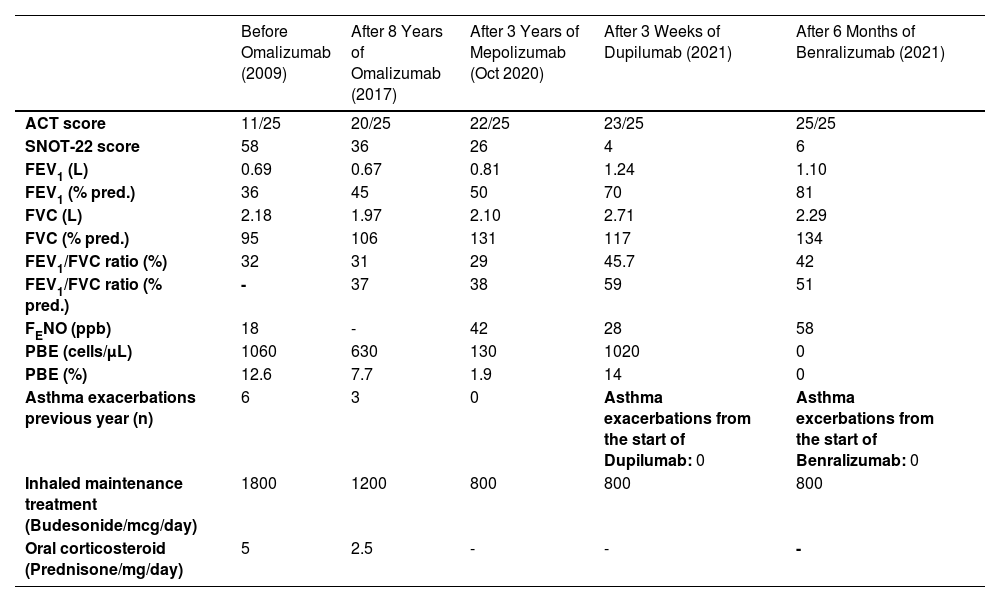
Table showing the improvement over time in functional pulmonary parameters, clinical scores, decrease in asthma exacerbations (OCS bursts previous year) and maintenance treatment,switching from one biologic to another.
ACT= Asthma control test, FEV1= Forced expiratory volume in the first second, FVC= forced vital capacity, FEV1/FVC ratio=Tiffeneau Index, FENO=Fractional exhaled nitric oxide, PBE=Peripheral blood eosinophils, SNOT-22=Sinonasal outcome test, % pred.= % predicted.
Different biologics can be prescribed in severe, uncontrolled, T2-high asthmatics. Based on surrogate biomarkers our patient showed a T2-high phenotype, while bronchial biopsies demonstrated a mixed inflammatory profile configuring a more severe disease.2,3 After prolonged Omalizumab treatment the patient could not suppress the risk of clinical worsening and couldn't interrupt OCS, leading to the switch to Mepolizumab. Currently, there are no clear recommendations neither for switching from one biologic therapy to another nor about the length of the washout period between drugs.4 Patients who failed Omalizumab treatment resulted in Real-life experiences to be good candidates for a successful anti-IL5 therapy.5
Mepolizumab seemed to be the correct treatment for our patient. However, recurrence of NPs, a well-known risk factor for poor asthma control and a cause of poor response to anti-IL-5 treatments, may cause physicians to switch between biologics.4 Dupilumab, effective on both severe asthma and NPs,6 was started leading to clinical improvement after only 3 weeks. Unfortunately, it was cautiously withdrawn after side effects occurred. Even if clinically mild, side effects significantly impact the quality of life, for example tachycardia in an old lady already symptomatic for asthma. A mild to moderate increase in PBE is expected but usually transient following Dupilumab. However, high and sustained PBE suggests close monitoring and investigation of eosinophilic-related morbidity.7
Finally, Benralizumab caused complete control of nasal and asthmatic symptoms with evidence of mild inflammatory sinonasal tissue reduction.
This clinical case shows that the appropriate biologic should be selected according to the dominant severe asthma phenotype. Surrogate biomarkers are currently used for disease phenotyping, however, overlap between T2 endotypes is often evident, complicating the choice for the best treatment. In some specific cases, performing bronchial biopsy gives a more detailed profile of the underlying inflammatory pathway. Today the identification of treatable traits, more susceptible to a certain biologic, may guide the choice. The lack of biomarkers predictors of clinical response may justify the failure and a switch of treatment. An algorithm for appropriate switching has been proposed but its application in real life needs further observation.8
We evaluated 4 different biologics in the same patient, improving step-by-step from one biologic agent to another (Table 1). At the age of 82, she feels much better than when she was 69: FEV1, ACT and SNOT improved over time such as the burden of daily treatment.
We thank Miss. Laura Gibson for the English language revision. We thank Dr. Vitina Carriero and Dr. Francesca Bertolini for their kind contribution.
This clinical case was selected for “Lungs on Fire: airway diseases” (session 421) at the ERS Virtual International Congress 2021, 5-8 September.