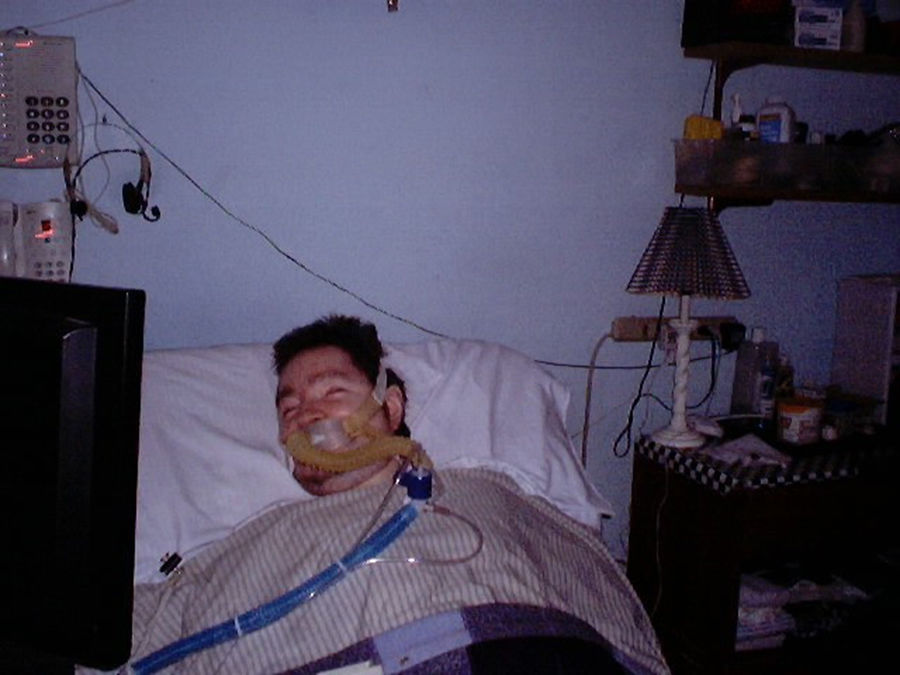
This case series of five patients with Duchenne muscular dystrophy demonstrates the nutritional advantages of instituting noninvasive intermittent positive pressure ventilatory support via 15mm angled mouthpieces to relieve tachypnea and provide more time to swallow food safely. In each case weight loss was reversed.
Up to a 10-year survival advantage has been reported by using up to continuous (C) noninvasive ventilatory support (NVS) over that which occurs by resort to tracheostomy mechanical ventilation (TMV) for patients with Duchenne muscular dystrophy (DMD).1 In addition, there are enormous cost and quality of life advantages by definitively avoiding tracheotomy.2 Occasionally, before NVS is introduced to treat symptomatic hypoventilation, patients present with dysphagia and tachypnea which results in decreased appetite and weight loss due at least in part to insufficient time to swallow safely. This case series demonstrates that mouthpiece NVS (Fig. 1) can be used to increase breath volumes and swallowing time instead of conventional resort to gastrotomy.
In DMD progressive generalized muscle weakness results in dysphagia and eventually in hypercapnia.3–5 Decreased tidal volumes cause a compensatory tachypnea to maintain adequate minute ventilation. Swallowing is only safe during expiratory pauses but tachypnea reduces swallowing time to less than 1 second, which renders swallowing unsafe, often occurring during inspiratory pauses. The impaired swallowing and hypercapnia can cause depression and decreased appetite along with malnutrition and weight loss.6 Conventionally, gastrotomy is usually performed along with tracheotomy.7 However, both can detract from quality of life and the tracheostomy tube can further impair swallowing by transpiercing the strap muscles in the neck and rendering cough ineffective by preventing the glottis closure necessary for pre-cough thoracoabdominal pressure build-up. When dysphagia is exacerbated by indwelling tracheostomy tubes the incidence of aspiration increases and can remain elevated after tube removal even for patients who apparently had intact bulbar-innervated musculature prior to tracheotomy.8,9
This case series presents an alternative to tracheotomy and premature resort to gastrotomy to reverse weight loss for patients with DMD. All five cases initially used mouthpiece NVS (Fig. 1) only for meals before eventually requiring it continuously. Four patients received 800–1500ml volume preset ventilation and one 20cm H2O pressure preset ventilation delivered through active ventilator circuits from portable ventilators. No expiratory positive airway pressure or positive end-expiratory pressure was used. None received glucocorticoids for fear of side effects. Cases 1 through 4 never underwent tracheotomy. All received dysphagia therapy and were placed on pureed soft food diets which did not change once mouthpiece NVS was begun. No patients received “energy supplements” nor were any referred to nutritionists since it was clear what was causing the weight loss.
Case reportsCase 1At 13½ years of age a wheelchair dependent patient since age 6 weighed 91lb. and complained of weight loss, decreased appetite, and choking. His respiratory rate varied from 38 to 45 breaths per minute. He was placed on volume preset mouthpiece NVS (Fig. 1) at 1200ml during meals which decreased his respiratory rate (RR) to 8–10/min. His vital capacity (VC) at that time was 540ml (22% of normal). He immediately reported improved appetite, easier swallowing, and relief of choking. Over the next 12 months he gained 11lb. He eventually became CNVS dependent at 14 years of age and remained so for the next 27 years until his death at age 41 from sepsis secondary to a skin decubitus ulcer.
Case 2A 21-year old, wheelchair dependent patient since age 12, complained of a 21lb. weight loss over a 3-year period associated with choking, dyspnea during meals, and decreased appetite. His RR varied from 35 to 48/min. He was placed on volume preset mouthpiece NVS at 1300ml only during meals. He immediately reported relief of choking, better appetite, and gained 9lb. over the next 6 months. One year later, he presented with morning headaches and fatigue, and began sleep NVS via lip cover interface as for Case 1. By age 26 he was overweight and CNVS dependent. His VC had decreased to 220ml by age 33. He died suddenly at age 46.
Case 3A 17-year old boy, wheelchair dependent since age 7, complained of loss of appetite and 13lb. weight loss over a 1-year period. His RR varied from 30 to 47/min. He was placed on 800ml volume preset NVS with a back-up rate of 10/min during meals and subsequently during sleep several months later. Meal use decreased his RR to 12/min. He immediately reported better appetite and had a 9lb. weight gain over the next 7 months. By age 18 with a VC of 320ml and daytime end-tidal CO2 of 60mm Hg he became CNVS dependent and remained so until age 27 when, with severe cardiomyopathy, he died from cardiovascular instability during relief of a pressure pneumothorax.
Case 4A 24-year old patient, wheelchair dependent from age 9, complained of depression, loss of appetite, weight loss, and difficulty eating and swallowing. Despite hypercapnia he refused sleep NVS but used mouthpiece NVS during meals. This relieved tachypnea and permitted weight gain. At age 28 with a VC of 500ml he began sleep NVS. At age 31 with 330ml of VC he required CNVS. He died at home at age 33 from a mucus plug during an upper respiratory tract infection after refusal to seek medical attention.
Case 5An out-of-state DMD patient, wheelchair dependent from age 10, complained of difficulty swallowing and weight loss over a 2-month period. He began mouthpiece NVS during meals at age 29. Although his parents reported by phone that he gained weight, he did not return for a follow-up evaluation. The next year he extended daytime mouthpiece NVS use to on and off all day and used it for sleep as well but without lip cover retention. He became CNVS dependent through age 34. With no lip seal use during sleep, excessive air leakage out of the mouth deranged his sleep and he underwent elective tracheotomy after consultation with local physicians. Malnutrition developed because of dysphagia exacerbated by tracheostomy tube restriction of the muscles of deglutition. A gastrostomy tube was placed shortly thereafter.
DiscussionThese five typical DMD patients developed dysphagia and tachypnea which compromised safe swallowing. Although not yet using NVS for symptomatic hypoventilation, mealtime mouthpiece NVS was instituted to slow RR and increase breath volumes to allow more time to swallow food safely. All five patients instantly mastered mouthpiece NVS. In our experience less than 1 in 20 patients with DMD are too mentally impaired to grab a mouthpiece for NVS. A more common problem is that the lips can become too weak to grab the mouthpiece NVS and so nasal NVS is used with no or a very low back-up rate during meals.
Typically, tachypneic patients with neuromuscular disorders who develop dysphagia, lose weight, and undergo percutaneous endoscopic gastrotomy (PEG). Since this is usually done under general anesthesia with the patient intubated, and the patients have little respiratory reserve, many if not the majority undergo tracheotomy as well.10 Of seven patients in the study of Lofaso et al., six underwent elective tracheotomy and the authors reported “improvement in bolus size, increased swallow time, increased number of swallows per bolus and less perceived respiratory difficulty during swallowing, possibly due to correction in hypercapnia, decreased work of breathing, and eliminating the need for patients to coordinate breathing and swallowing.”7 While this is certainly true, it can be accomplished without tracheostomy tubes that, themselves, impede swallowing by fixing the trachea against the skin to impair strap (swallowing) muscle function, decrease laryngeal movement to uncoordinated closure, and cause loss of protective reflexes as well as proprioception.9 Open tracheostomy tubes also increase aspiration by decreasing subglottic pressure, with 80% of patients having abnormal modified Barium swallow studies and 50% aspirating despite having taken all food by mouth pre-tracheotomy.11
Kirshblum et al. reported that 73.8% of spinal cord injured (SCI) patients with no premorbid dysphagia but with tracheostomy tubes aspirated during videoflouroscopic swallowing studies.9 Elpern et al. reported that 50% of such patients aspirate even if the cuff is fully inflated and 77% had silent aspiration.8 In one SCI study, tracheostomy on admission to a rehabilitation center was the greatest predictor of dysphagia9; with 38% of patients with tracheostomy tubes at rehabilitation admission aspirating, 20% still aspirating at rehabilitation discharge, and 7.5% continuing to aspirate even years after decannulation.9 Forty-two percent of SCI patients with tracheostomies who did not use ventilators also had dysphagia. Thus, swallowing impairment and aspiration can persist even after decannulation9 and, according to Kirshblum et al., tracheotomy increases dysphagia risk.
In a case series, none of 242 CNVS dependent DMD patients from 11 centers that extubate ventilator “unweanable” patients to CNVS had any patients who chose to undergo tracheotomy, and none needed the tubes.12–14 In another study of 135 patients who had depended on CTMV and CNVS for at least 1 month each, with all of the CNVS users having had access to mechanical insufflations–exsufflation (MIE), all preferred the latter for swallowing as well as for safety, convenience, speech, appearance, comfort, and overall.15 Thus, patients with DMD can become dependent on CNVS or CTMV, but over 70% of 28 patients over age 40 and dependent on CTMV received all nutrition via gastrostomy tubes compared to 1 of 27 patients over age 40 and dependent on CNVS in a recent Japanese study.10 The figure of 70% undergoing gastrotomy along with tracheotomy is likely accurate across the United States in general where there are few centers that offer CNVS. Likewise, in another study of 64% (16 of 25) of DMD patients who underwent concomitant tracheotomy and gastrotomy, three of the eight who were decannulated subsequently also had their gastrostomies closed.16
The recourse to both tracheotomy and gastrotomy is in marked contrast to 18 of 108 (15%) CNVS dependent patients with DMD who have gastrostomy tubes.16 Similarly, Wollinsky reported that only 3 of 21 (14.3%) DMD CNVS users required gastrostomy tubes.17 The high rate of oral intake in these study populations is explained in part by their use of NVS during the meals and the fact that CNVS decreases caloric needs for respiratory function.17
Remarkably, of 45 CNVS dependent patients with DMD in Japan, mean age 31.2±7.0 (range, 17–45) years, 43 (95.6%) were receiving all nutrition by mouth despite being CNVS dependent for 7.2±4.7 years (maximum 16.4 years). None required tracheotomies. Thus, only 2 of 45 CNVS dependent patients (4.4%) with DMD lost the ability to take food by mouth and they are 33.2 and 36.1 years of age, respectively.18 All used CNVS, therefore, NVS was used during meals as reported in this case series.
Typically, patients with DMD who use sleep NVS whether via lip cover, nasal, or oronasal interface, eventually become dyspneic when trying to discontinue it in the morning.19 At that point they transition to NVS via a mouthpiece fixed adjacent to their mouths (Fig. 1). They typically progress to requiring CNVS, including during meals.19 Some, whose lips become too weak for mouthpiece NVS, switch to nasal prong interfaces during daytime hours including during meals with back-up ventilator rates as low as 0 to permit the patients to trigger deep insufflations to facilitate eating without an interfering back-up rate. This can provide adequate minute ventilation with only 3 or 4 assisted breaths. Typically, 1200–1500ml volumes are administered to facilitate 6000ml minute ventilation with 12–15s between assisted breaths to facilitate safe swallowing. The NVS also facilitates speech and coughing to further decrease aspiration risk. As we see here, some patients begin mouthpiece NVS only to facilitate eating and speech.
Financial disclosureThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interestThe authors have no conflicts of interest to declare.