Osimertinib is recommended as first-line therapy for patients with advanced lung adenocarcinoma harboring epidermal growth factor receptor (EGFR) mutations.1 Compared with other EGFR-tyrosine kinase inhibitors (TKIs), treatment with osimertinib causes a high incidence of leukopenia, particularly lymphocytopenia. We report a case of pneumocystis jirovecii pneumonia (PjP), which seemed to occur after osimertinib-induced lymphocytopenia and resultant immunosuppression.
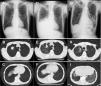
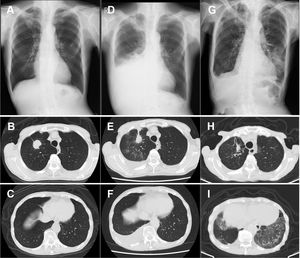
An 86-year-old man with a past smoking history (3 pack-years) was diagnosed with stage ⅠA3 right upper lung adenocarcinoma (cT1cN0N0) in March 2021 (Fig. 1A–C). Instead of curative surgical resection, he opted for heavy ion radiotherapy in May 2021, with significant shrinkage of the nodule. However, his disease recurred in August 2021, and he presented with right carcinomatous pleuritis (Fig. 1D–F). Based on the cytological detection of an adenocarcinoma harboring the L858R EGFR mutation, osimertinib 40 mg daily was initiated as first-line therapy in September 2021 [peripheral blood lymphocyte (PBL): 1700 cells / mm3]. Beginning in November 2021 (PBL: 700 cells / mm3), the drug dose was increased to 80 mg daily. Although he had a good performance status with grade 2 dermatitis, his PBL further decreased in November 2021 (400 cells / mm3). Thereafter, he noticed gradual worsening dyspnea on exertion and was eventually admitted to our hospital in December 2021 (on day 118 from the onset of osimertinib treatment). The chest-computed tomography revealed remission of the right pleural effusion, although new bilateral ground-glass opacities were noted (Fig. 1G–I). Further diagnostic studies, such as bronchoscopic examination, could not be conducted because the patient was hypoxemic. We suspected that the lesions were consistent with osimertinib-induced interstitial lung disease (ILD), thus we discontinued osimertinib and started intravenous prednisolone (40 mg daily). The laboratory data on admission showed decreased PBL (200 cells / mm3), and HIV serology was negative. His serum β-D-glucan was elevated (174 pg/ml), serum aspergillus galactomannan antigen was negative, and sputum PCR for Pneumocystis jirovecii DNA was positive on day 4 of hospitalization; all of which supported the diagnosis of PjP. He was treated with sulfamethoxazole-trimethoprim, which improved his symptoms and lung shadow dramatically. We are now planning to re-start osimertinib at a lower dose, which would not cause severe PBL reduction.
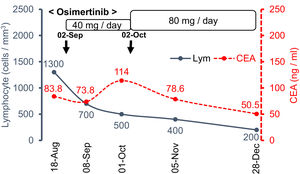
Osimertinib is a third-generation EGFR-TKI and a good treatment option in patients with EGFR-mutated advanced lung adenocarcinoma.1 Other than the rare but sometimes lethal ILD, EGFR-TKIs are widely considered as relatively safe and well tolerated drugs compared with previous cytotoxic drugs.1,2 Among various EGFR-TKIs, osimertinib has a unique adverse effect of early reductions in leukocyte and platelet counts; most of which usually stabilize over time and remain above the lower limit of normal thereafter. Lymphocytopenia is more common and was found in 62% of the included patients, although most were mild or moderate in severity and usually did not lead to dose interruption or discontinuation (grade 3 or higher lymphocytopenia is reported to be 6.1%).3 In this case, the gradual increase in drug dose was inversely correlated with the severity of the lymphocytopenia (Fig. 2). Compared with the absolute peripheral blood neutrophil counts, the PBL count usually remains low in priority when assessing for adverse events; therefore, it might be necessary to pay attention to sustained lymphocytopenia.
Pneumocystis jirovecii infections are typically seen in patients on steroid treatment and in immunocompromised hosts with impaired cell-mediated immunity, such as patients with human immunodeficiency virus infection and hematologic neoplasms. PjP has also been reported to develop among patients with lung cancer, and an analysis by Lee et al. reported radiotherapy and lymphopenia (< 1,000 cells / mm3) as significant risk factors for PjP development.4 Several TKIs such as idelalisib cause higher incidence of PjP.5 However, there have only been a few reports concerning the relationship between PjP and EGFR-TKIs such as gefitinib,6 erlotinib,7 and afatinib.8 Considering that the medical history of the patients in these reports suggested the presence of the above-mentioned risk factors for PjP, such as corticosteroid use 6,7 or post-chemoradiation therapy status,8 it is unclear whether EGFR-TKIs are directly associated with the occurrence of PjP or if the prior immunocompromised status was more important. In 2021, Emilie et al. reported two cases of PjP during treatment with osimertinib 9; neither of the subjects had any risk factors, and the author suggested the necessity of PjP prophylaxis. Since lymphocytopenia is a unique adverse event of osimertinib, it might be possible that the PjP occurred as an opportunistic infection secondary to the osimertinib treatment
In conclusion, this is a rare but important report of PjP, due to osimertinib-induced lymphocytopenia. Since the discontinuation of EGFR-TKIs can sometimes cause “flares” of the disease (accelerated disease progression) and result in poor prognosis,10 physicians must be careful in differentiating drug-induced ILD and other opportunistic infections such as PjP during osimertinib-induced lymphocytopenia.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics approvalThis study was exempt from ethics review board approval by the Institutional Ethic Committee.
Informed consentAppropriate written informed consent was obtained for publication of this case report and accompanying images.
We would like to thank Editage (www.editage.jp) for the English language editing.