Nuclear protein in testis (NUT) carcinomas are exceptionally rare and aggressive, with unknown incidence. Although these carcinomas were initially recognized as occurring almost exclusively in young patients, recent literature suggests that NUT carcinomas (NCs) may affect both sexes equally, with an extensive age range.1–3
NC is a rare and poorly differentiated carcinoma. In nearly 70% of cases, the NUT gene (15q14) is fused to the BRD4 gene (19p13.1), resulting in a BRD4-NUT fusion gene that leads to global hypoacetylation and transcriptional repression of differentiation genes.1,3,4 Hereditary or environmental factors are not related.5 Almost 51% of NCs originate from thorax, 41% from head and neck, 6% from bone or soft tissue, and 2% from other organs.1 The “midline” term is no longer included in the “WHO classification of tumours”.6 Regardless of NC predisposition to involve midline structures, were described cases of carcinomas arising from the bladder, pancreas, salivary glands, and bones.4,7
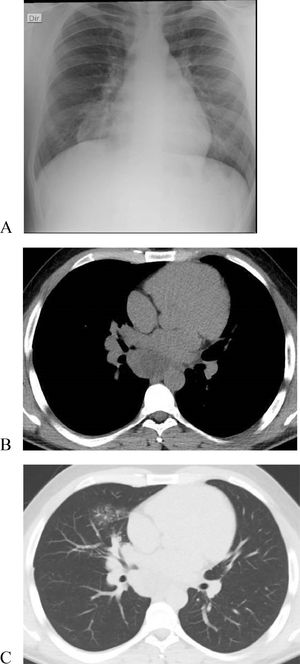
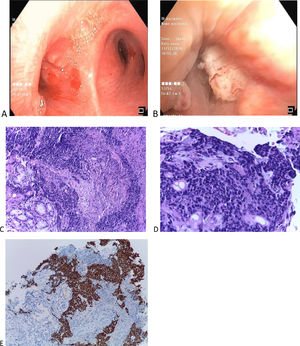
We report a case of a 25-year-old male with no relevant medical history or smoking habits who sought medical care for a subacute cough lasting four weeks. Clinical examination was unremarkable. Chest X-ray showed right mediastinal enlargement, and chest CT evidenced a 64 × 50 mm infracarinal mediastinal mass and a right middle lobe consolidation (Fig. 1A–C). Videobronchofibroscopy revealed small nodular lesions on the left main bronchus entrance and middle lobe bronchus narrowing due to a “cauliflower” lesion (Fig. 2A-B). Bronchial biopsies, endobronchial ultrasound, and transbronchial needle aspiration of station 7 lymph nodes were performed. Bronchial and lymph node samples showed solid to trabecular pattern neoplasia with keratin pearls and abrupt keratinization. Immunohistochemistry was positive for NUT protein (C52B1; 1:40; Cell Signaling Technology, Inc., USA), p40, p63, CK5, CK34B12 and CD56 (Fig. 2C–E), and negative for TTF-1, Napsin-A, synaptophysin, chromogranin and CD34. FISH and RT-PCR were not performed.
(Original images) (A-B) Videobronchofibroscopy images: (A) Small nodular lesions on the left main bronchus entrance (B) Middle lobe bronchus narrowing due to a “cauliflower” lesion. (C) Respiratory mucosa fragments, documenting infiltration by solid to trabecular pattern neoplasia of small, cuboidal cells with granular chromatin and generally absent nucleoli, with scant eosinophilic cytoplasm. Foci of necrosis and abrupt keratinization and keratin pearls formation are identified (C-HE 10x, D-HE 20x). (E) Immunohistochemical study positive, strong and diffuse for NUT (HE 10x). (Original images).
Subsequent evaluation, including PET/CT, revealed an extensive middle lobe infiltrative lesion (94 × 69 mm); right broncho-hilar, infracarinal (with left main bronchus projection), oesophageal, and diaphragmatic adenopathies; a hepatic lesion; a right supra-renal nodule; and osteomedullary lesions. An IV-B stage thoracic NC (TNC) was assumed, and the patient is presently enrolled in a clinical trial of a bromodomain and extraterminal (BET) inhibitor.
The clinical signs of TNCs are nonspecific, including dyspnoea, haemoptysis, chronic cough, nausea, and pain from bone metastasis.5 In a review of seven TNCs, all patients complained of cough lasting for more than a month.2 Usually, symptoms relate to tumour location and mass effect. Because of the tumour's rapid development, constitutional symptoms are not frequent.7 Usually, TNCs are accompanied by distant metastases at the time of diagnosis.5 Bones are the most common site of extrathoracic involvement, and it is thought that the majority of these patients do not live long enough to develop brain metastases.2,7 A recurrent question has been whether NCs arise primarily in the lung, or secondarily involve the lungs due to aggressive growth within the mediastinum5. In WHO 5th edition, were reclassified as thoracic NUT carcinomas.6
The histologic features are unspecific; it is poorly differentiated, with or without squamous differentiation with a monomorphic appearance. Abrupt keratinization is suggestive but not pathognomonic of this entity.8 NCs can mimic other undifferentiated neoplasms, such as germ cell tumours, Ewing's sarcoma or lymphoma.7 In the reported case, one of the evaluated neuroendocrine (NE) markers (CD56) was positive, highlighting an unusual finding and the nonspecific character of NE marker expression, underlining the need to maintain a broad differential diagnosis when approaching high-grade carcinomas.9 Thoracic tumours with aggressive expression and pleomorphic/undifferentiated carcinomas should warrant prompt evaluation for NUT translocation.1 Diagnosis is based on the demonstration of the NUT genetic rearrangement, either by NUT immunohistochemical analysis, FISH or RT-PCR.1,7,8 In imaging descriptions of TNCs, they usually present as large masses with lymphadenopathy and pleural involvement, with no side/lobar predilection1 — relatively nonspecific but consistent features. In seven assumed pulmonary NCs, the most constant imaging finding was a large unilateral lesion involving the lung, pleura and mediastinal lymph nodes.2
The diagnosis is frequently overlooked. Several factors contribute to underdiagnosis, namely insufficient reporting, age bias and nonspecific morphology.7
Currently, there are no standardized guidelines aimed at treating NC.1,2 Surgical options are limited by its fast growth. Radiotherapy frequently has a palliative role, and NC usually does not respond to chemotherapy or immunotherapy regimens.7
Further possible therapeutic options include histone deacetylase inhibitors and BET inhibitors.5 BET inhibitors bind to the BRD4-NUT chromatin binding site, inhibiting gene activation, and at least three studies have reported improved survival in NC patients.1 However, the outcome has been invariably fatal, and the average survival rate is six to seven months after diagnosis.7
Regardless of the patients’ age and the absence of risk factors, NC should be included in the differential diagnosis of thoracic masses with a rapidly progressive clinical course.2 The outcome is poor, as NCs are refractory to conventional therapy, and promising targeted therapies are currently under testing. Both clinicians and pathologists should be aware of this rare entity to make the proper diagnosis and establish effective treatment algorithms.
Author contributionsStudy conception and design: Ana Alfaiate, Vera Clérigo.
Data acquisition: Ana Alfaiate, Carolina Padrão, Vera Clérigo, Ivone Fernandes.
Data analysis and interpretation: Ana Alfaiate, Carolina Padrão, Vera Clérigo, Ivone Fernandes.
Drafting of the manuscript: Ana Alfaiate, Vera Clérigo.
Critical revision of the manuscript for important intellectual content: Carolina Padrão, Ivone Fernandes.
All authors had access to the data and played a role in writing this manuscript.
We acknowledge António Alves MD for making the diagnosis of NUT carcinoma; without his knowledge, this article would not be possible.
We acknowledge the Instituto Português de Oncologia Francisco Gentil, Lisboa for performing the NUT immunohistochemical analysis.