Noninvasive respiratory support (NRS) has become a valuable tool in COVID-19 acute respiratory failure (ARF) management. However, there is a lack of consensus in for or against its use due to the risks of intubation delay and aerosols environmental contamination.1 Strategies to mitigate NRS iatrogenic risks and healthcare workers infection have been suggested.2,3
Despite efforts to increase ICU resources, to cope with patient influx, general wards worldwide were converted in respiratory intermediate care units (RICU), where patients in need for NRS were treated.4,5 Our goal was to evaluate the Portuguese reality regarding safeness and outcomes of NRS in COVID-19 ARF in a non-ICU setting.
Adult patients with COVID-19 pneumonia needing NRS [high flow nasal cannula (HFNC), continuous positive airway pressure (CPAP) and/or noninvasive ventilation (NIV)], treated in a RICU, were prospectively enrolled from November 18th, 2020 to February 18th, 2021. Institutional review boards authorised the study (n. 22/2021). Informed consent was waived.
High flow nasal cannula was instituted if arterial oxygen partial pressure to fractional inspired oxygen (PaO2/FiO2) ≤ 200 mmHg with FiO2 > 0.4, respiratory rate (RR) ≥ 25cpm and/or sings of respiratory distress. Continuous positive airway pressure and NIV was started if SpO2 <92%, RR ≥ 25bpm and/or sings of respiratory distress in patients with HFNC failure or ad initium if HFNC failure was expected. Arterial oxygen partial pressure to fractional inspired oxygen ratio was accessed before NRS institution. In patients with HFNC failure who increased support to CPAP/NIV, the PaO2/FiO2 considered was the one before CPAP/NIV. Noninvasive respiratory support settings were adjusted to maintain SpO2 92–96%. Continuous positive airway pressure and NIV was delivered by oronasal mask. All patients had a “do intubate” (DI) or “do not intubate” (DNI) order defined at admission. Baseline patient's characteristics were compared according to NRS technique (HFNC vs. CPAP/NIV). The success rate was defined by invasive mechanical ventilation (IMV)-free survival. Several outcomes were recorded: length of stay, complications, need for endotracheal intubation (ETI) and mortality. The association between NRS and outcomes was calculated using a logistic regression model adjusted for age, DNI order and PaO2/FiO2. A two-sided test of <0.05 was considered statistically significant.
190 out of 1748 hospitalized patients were treated with NRS in RICU. Table 1 summarizes patient's characteristics and treatment.
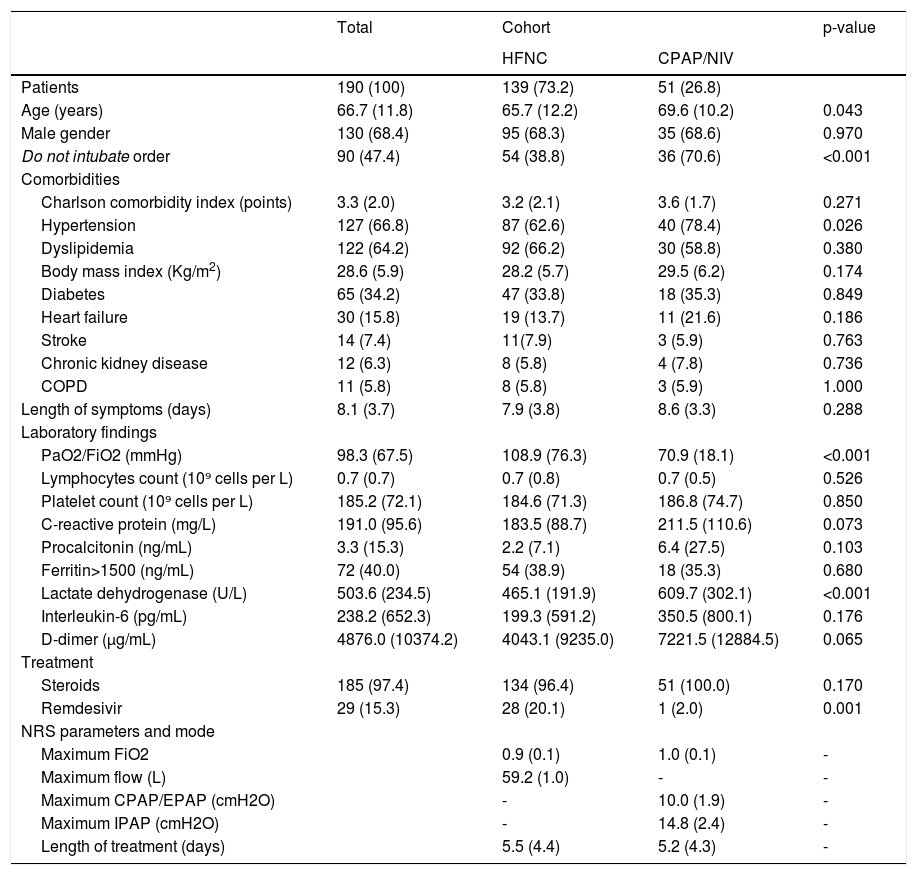
Demographic and clinical characteristics of the study population and according to cohort.
Data are presented as number (percentages) or mean (standard deviation) as appropriate. HFNC: high flow nasal cannula; CPAP: continuous positive airway pressure; NIV: noninvasive ventilation; COPD: Chronic obstructive pulmonary disease; PaO2/FiO2: arterial oxygen tension /inspiratory oxygen fraction. NRS: noninvasive respiratory support. EPAP: expiratory positive airway pressure. IPAP: inspiratory positive airway pressure.
50 patients needed ICU admission, 25.2% in HFNC group and 29.4% in CPAP/NIV group (p = 0.366). Considering only DI patients, 42.0% were escalated to ICU. Time from NRS to IMV was 3.5 ± 3.3 and 3.9 ± 2.8 days in survivors and deceased patients, respectively (p = 0.724). In-RICU and in-hospital mortality were 24.2% and 36.3%, respectively. In DI patients, there were no deaths in RICU and in-hospital mortality was 16.0%. In DNI patients, mortality rate was 50.0% and 58.9% in-RICU and in-hospital, correspondingly. Globally, 63.7% survived hospitalization, 53.2% exclusively treated with NRS techniques in RICU.
Table 2 illustrates outcomes according to NRS. In HFNC group, 61.9% were successful weaned and 27.1% needed ETI. The success rate in DI and DNI patients was 68.2% and 51.9%, respectively (p < 0.001). In-hospital mortality was 27.3% (14.1% for DI vs. 48.1% for DNI patients, p < 0.001). In CPAP/NIV group, CPAP was the preferred technique (56.9%). 29.4% were successfully weaned and 53.3% needed ETI. The success rate in DI and DNI patients was 40.0% and 25.0%, respectively (p = 0.005). In-hospital mortality was 60.8% (26.7% in DI vs. 75.0% in DNI patients, p = 0.001).
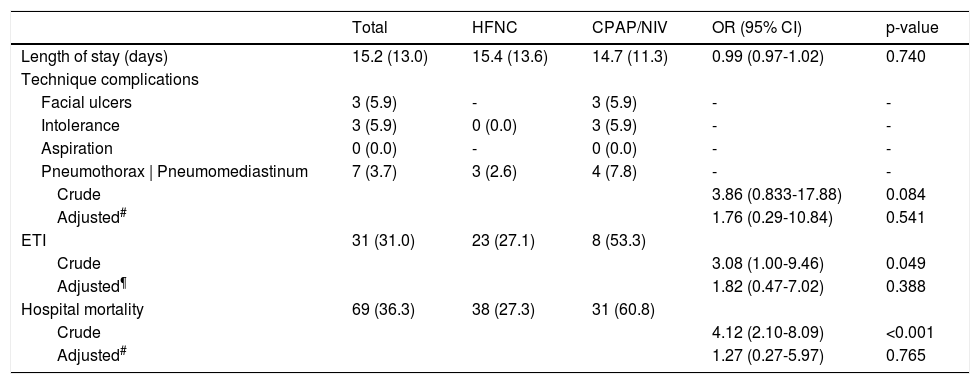
Clinical outcomes and relative probability according to noninvasive respiratory support technique.
| Total | HFNC | CPAP/NIV | OR (95% CI) | p-value | |
|---|---|---|---|---|---|
| Length of stay (days) | 15.2 (13.0) | 15.4 (13.6) | 14.7 (11.3) | 0.99 (0.97-1.02) | 0.740 |
| Technique complications | |||||
| Facial ulcers | 3 (5.9) | - | 3 (5.9) | - | - |
| Intolerance | 3 (5.9) | 0 (0.0) | 3 (5.9) | - | - |
| Aspiration | 0 (0.0) | - | 0 (0.0) | - | - |
| Pneumothorax | Pneumomediastinum | 7 (3.7) | 3 (2.6) | 4 (7.8) | - | - |
| Crude | 3.86 (0.833-17.88) | 0.084 | |||
| Adjusted# | 1.76 (0.29-10.84) | 0.541 | |||
| ETI | 31 (31.0) | 23 (27.1) | 8 (53.3) | ||
| Crude | 3.08 (1.00-9.46) | 0.049 | |||
| Adjusted¶ | 1.82 (0.47-7.02) | 0.388 | |||
| Hospital mortality | 69 (36.3) | 38 (27.3) | 31 (60.8) | ||
| Crude | 4.12 (2.10-8.09) | <0.001 | |||
| Adjusted# | 1.27 (0.27-5.97) | 0.765 |
Data are presented as number (percentages) or mean (standard deviation) as appropriate. HFNC: high flow nasal cannula; CPAP: continuous positive airway pressure; NIV: noninvasive ventilation; ETI: endotracheal intubation.
During the study period, our hospital received 10% of national cases of COVID-19. 10.9% were treated in RICU and 50% were successfully managed with NRS.
Considering patients with DI order, ICU admission was avoided in almost 60% and only a third needed ETI. Delays in ETI have prognostic impact and it has been suggested that NRS might have contributed to the problem. Time to IMV was similar in survivors and deceased patients. Moreover, casualties in RICU were not reported and in-hospital mortality was lower than previously described5,6, supporting strategy safeness.
In-hospital mortality was 36.6%, higher than in other series.5,7,8 It is important to note that, in our sample, ARF was severe and almost 50% were DNI patients. Interestingly, 41% of DNI patients survived hospitalization supporting the role of NRS in patients with therapeutic ceiling.
We report a success rate of 62% in HFNC group, with a survival greater than 50% in DNI patients. In CPAP/NIV treatment group, success was achieved in 30%. Patients in this group were older, had more severe ARF and 71% were DNI. Mortality rates in patients treated outside-ICU, in whom NIV was the therapeutic ceiling, reached 76% in previously reports, which is in line with our findings.6,7,9 Nevertheless, CPAP/NIV prevented IMV in 40% of the candidates, with mortality rates similar to published data.10
The difference in ETI and mortality rates between groups disappeared after adjustment for confounders, emphasising that outcomes don't rely on the NRS technique. Complications were infrequent. Pneumothorax/pneumomediastinum is reported in 2% of ICU treated patients.11 Barotrauma occurred in both groups equally after adjustment for confounders, suggesting that underling disease severity might play a role.
Our study has limitations: the decision to start on a specific NRS technique greatly relied on equipment availability and is single-centered with a limited sample.
To the best of our knowledge this is the first prospective study on NRS outside-ICU in COVID-19 pneumonia, conducted in one of the Portuguese regions that was most affected. Both HFNC and CPAP/NIV seem to be equally feasible and outside-ICU should be considered for selected patients in resource-constrained settings.
Contributions of the authorsLígia Rodrigues Santos, Rafaela Gonçalves Lopes, Ana Silva Rocha, Marta Dalila Martins, Teresa C. Guimarães, Mariana Meireles and Helena Vilaça collected the clinical and biological data from the files.
Mariana Meireles and Helena Vilaça, provided assistance for statistical analysis.
Lígia Rodrigues Santos, Rafaela Gonçalves Lopes, Mariana Meireles and Helena Vilaça wrote the manuscript.
Alice Castro, Mari Mesquita revised the manuscript
All the authors have checked the manuscript.
We would like to thank all healthcare professionals that have been working in RICU during this pandemic.
We would also like to thank the ICU team for all the support provided.