Chronic eosinophilic pneumonia (CEP) is a rare disease that is most common in women, typically non-smokers and with peak incidence in the third and fourth decades. Asthma precedes the onset of this disease by several weeks to years in up to two-thirds of the cases. CEP presents as a subacute onset of dyspnea and cough associated with systemic symptoms.1 Wheezing and crackles may be present at auscultation, and in many cases, mild hypoxemia can be detected.2 The diagnostic criteria consist of the presence of respiratory symptoms for at least two weeks: (1) a typical computer tomography (CT) scan of the chest with diffuse consolidation and/or ground-glass opacities with peripheral predominance, (2) alveolar eosinophilia commonly ≥ 40% in the bronchoalveolar lavage (BAL), (3) blood eosinophilia ≥ 1000/mm3, (4) and/or eosinophil infiltration in the lungs in the absence of an alternative diagnosis.2,3 The standard treatment consists of corticosteroids, and the response to these drugs is usually dramatic.1,4
In this report, we describe a case of eosinophilic pneumonia triggered by a severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2/COVID-2019) infection. A 51-year-old male, non-smoking insurance agent with a history of untreated dyslipidemia and uncontrolled asthma for the last year and a half was treated with formoterol and budesonide. The patient presented with myalgia, fever, and cough after having contact with a COVID-19 patient. The diagnosis of COVID-19 was confirmed by reverse transcription-polymerase chain reaction (rt-PCR). After one week at home the patient was asymptomatic, and during the quarantine time, he was only treated with acetaminophen as needed.
Two weeks later the patient started with progressive dyspnea (modified Medical Research Council [mMRC] scale: grade 2), wheezing, and productive cough. In the emergency department, he presented with fever (38 °C) and wheezing. A CT scan of the chest showed ground-glass opacities and crazy paving areas with < 10% lung involvement. At the time, these features were thought to be associated with the previous SARS-CoV-2 infection. The blood analysis showed leukocytosis 14,400/uL (normal 4000–11,000/uL, without blood cell differential) and increased C-reactive protein (CPR) 54.40 mg/L (normal < 0.5 mg/L). Given the possibility of bacterial infection, he was discharged home with cefixime for one week and azithromycin for three days.
After one week of antibiotic treatment, the patient did not feel better and returned twice to the emergency department. He denied a history of weight loss, anorexia, rhinosinusitis, paresthesia, sensibility changes, and skin lesions. The patient also denied consumption of illicit drugs or exposure to toxins. A CT pulmonary angiography was performed that excluded pulmonary embolism but showed worsening consolidations, ground-glass opacities, and crazy paving areas, predominantly peripheral, with about 15% of pulmonary involvement. The arterial blood analysis showed hypoxemia (pO2 60 mmHg) and blood analysis leukocytosis 17160/uL. At the last visit a white blood cell differential was performed showing 51.5% of eosinophils (8840/uL), confirmed by peripheral blood smear. The IgE level was 156 Ui/mL. He started empiric prednisolone 40 mg per day and was admitted to a ward for further diagnostic examination.
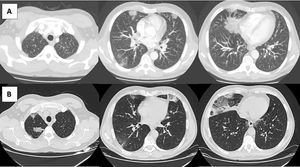
After one week, the CT scan showed migratory bilateral consolidations (Fig. 1). The immunological study and blood cultures were negative. Bronchoscopic evaluation of the airways was normal, and the immunologic study of BAL showed increased cellularity with eosinophilia of 94% and normal lymphocyte and neutrophil counts without microbiological isolates and negative cytology. Bilateral nasal polyps were found, and a biopsy confirmed it to be inflammatory lesions of chronic rhinosinusitis without granulomas, vasculitis, or signs of malignancy.
Imaging evolution of the patient. A) Computed tomography (CT) pulmonary angiography with consolidations and ground-glass opacities predominantly peripheral. B) CT scan of the thorax taken one week later, showing migratory bilateral consolidations that were predominantly peripheral and an increase in crazy paving areas and ground-glass opacities.
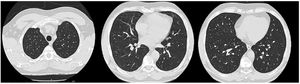
Given these findings, we assumed a diagnosis of eosinophilic pneumonia. The prednisolone dose was increased to 1 mg/kg per day with rapid clinical improvement. Two days later the patient was taken off oxygen and blood eosinophils levels were down to 0.1%. He was discharged home and started follow-up at an interstitial lung disease outpatient appointment. One month later the patient underwent a pulmonary function test that was normal, and a CT scan four months later showed complete resolution of the lung opacities (Fig. 2).
In this report, we describe a rare case of an eosinophilic pneumonia that was triggered by a SARS-CoV-2 infection. To the authors’ knowledge, only two cases of acute eosinophilic pneumonia associated with SARS-CoV-2 have been described in the literature.5,6 In our case, the patient presented a subacute onset of symptoms, initially presenting three weeks after the COVID-19 diagnosis with lung opacities and blood eosinophilia and then confirmed BAL eosinophilia. This patient was not taking any chronic medication and was not exposed to smoking, illicit drugs, or toxins and showed a dramatic response to corticosteroid treatment. This clinical case supports the already current evidence in which it is important to consider the development of interstitial lung disease in patients with COVID-19 including eosinophilic pneumonia. Despite the absence until now of a known mechanism for eosinophilic pneumonia in SARS-CoV-2, we believe that just as with other viruses, SARS-CoV-2 in rare cases can trigger an eosinophilic response.7
Patient's consentWritten informed consent was obtained for the publication of this case report and accompanying images.
The authors declare that no funding was received for this article.