Little is known about the light phenotype of SARS-CoV-2 pneumonia, which behaves in an unusual way, unlike other known respiratory diseases. We believe that the histopathological features of early COVID-19 could be considered the pathophysiological hallmark of this disease.
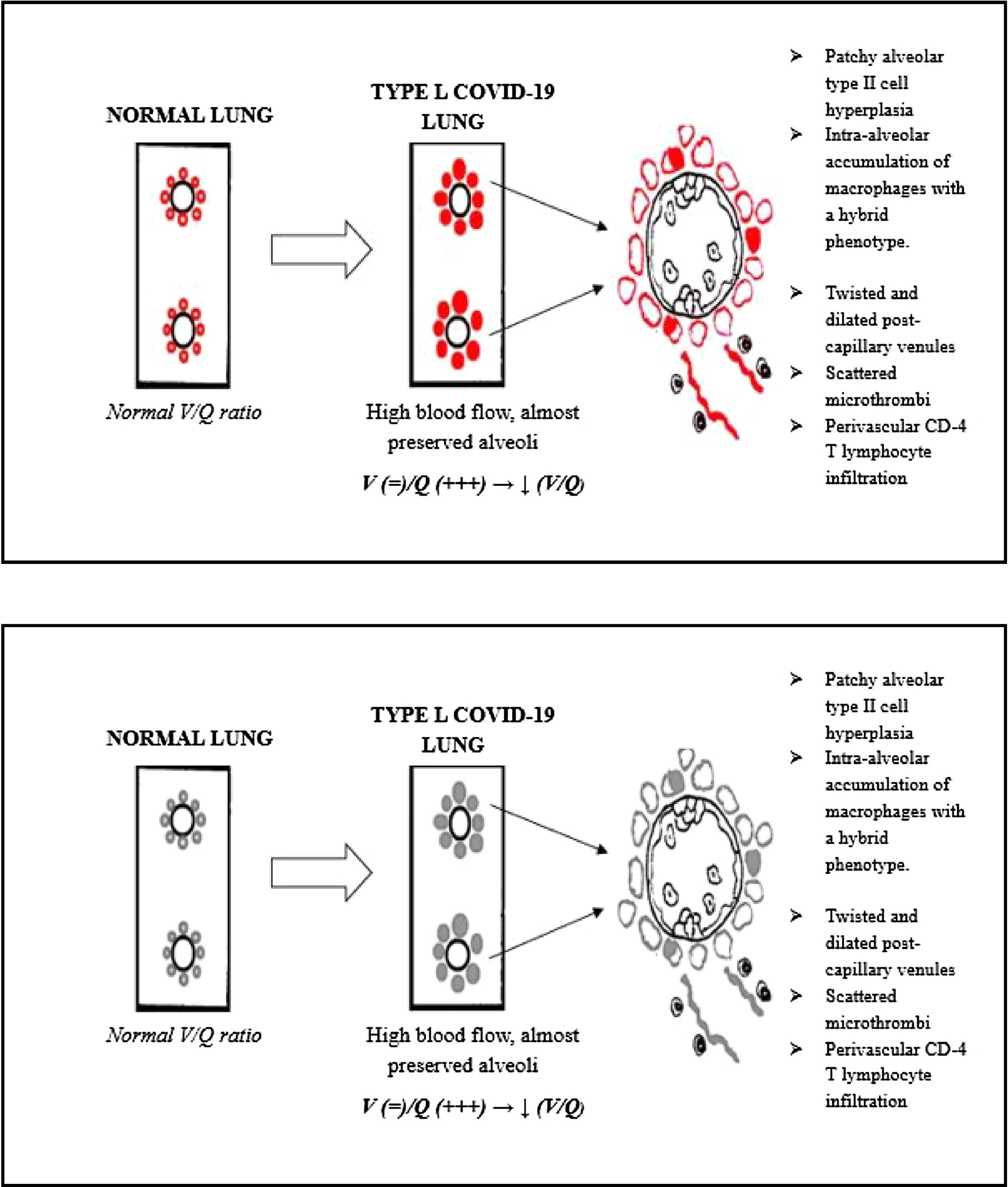
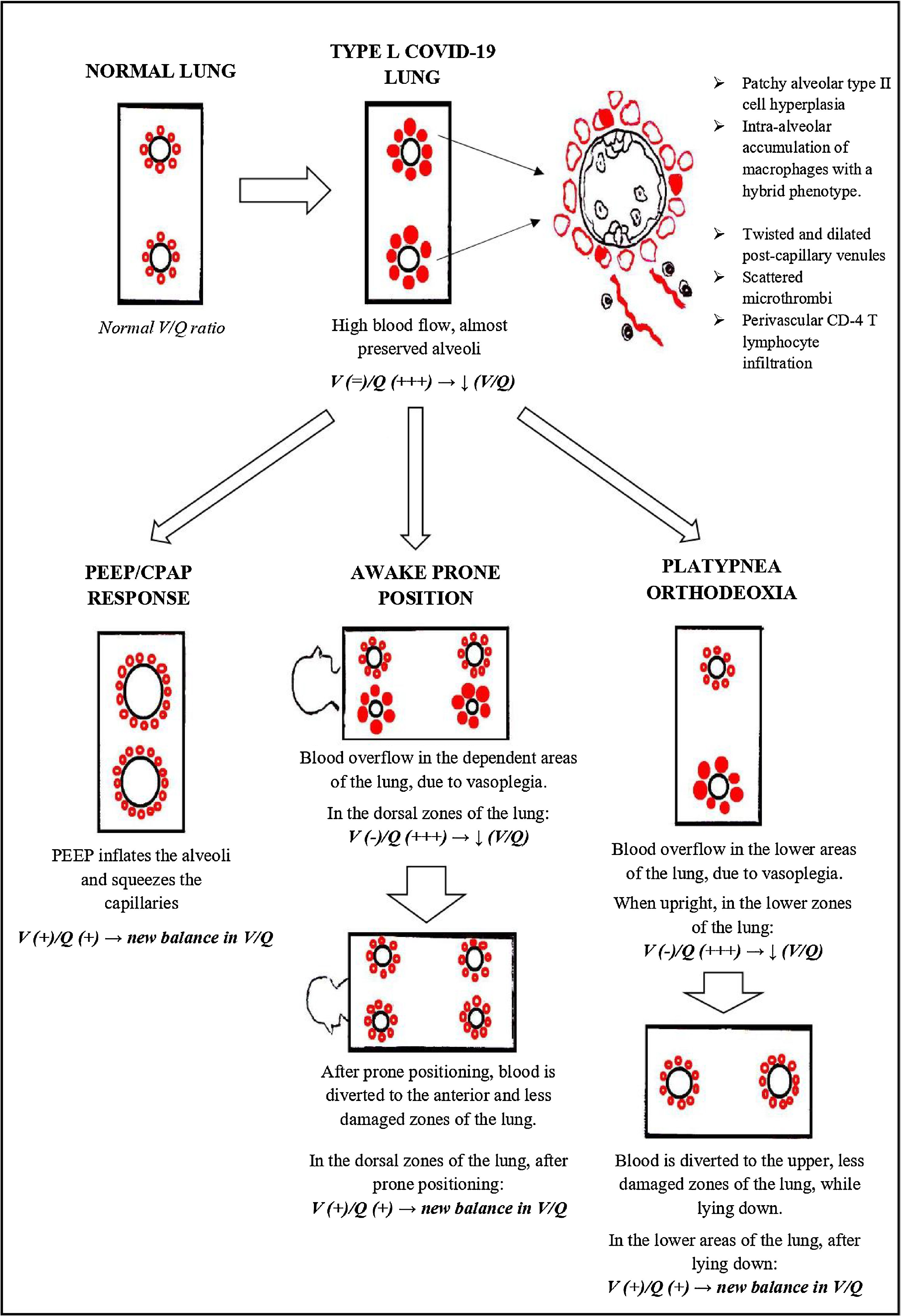
Lung cryobiopsies show almost pristine alveoli, enlarged/hyperplasic alveolar capillaries along with dilatation of the post capillary pulmonary venules. Hypoxemia could therefore be explained by a reduction of the normal V/Q ratio, due to blood overflow around well ventilated alveoli.
This could clarify typical manifestations of type L COVID-19, such as happy hypoxemia, response to awake prone positioning, response to PEEP/CPAP and platypnea orthodeoxia.
In early December 2019, the first cases of a pneumonia of unknown origin were identified in Wuhan, the capital of Hubei province in China. The pathogen responsible for this new disease was identified as a novel member of the RNA betacoronavirus family and was named Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), due to its similarities with the SARS-CoV, the virus responsible for the SARS epidemic in 2002–2003, and Middle East Respiratory Syndrome virus (MERS-CoV). The disease caused by SARS-CoV-2 infection was thereafter labeled as “coronavirus disease 2019” (COVID-19).1,2
COVID-19 has a wide spectrum of clinical severity. Mild disease is observed roughly in 81% of patients, whereas severe or critical forms are detected in 14 and 5% respectively.3 Severe and critical cases usually present with bilateral interstitial pneumonia that apparently fits in with the Berlin definition of Acute Respiratory Distress Syndrome (ARDS).4
Italy was the first western country to be involved in the COVID-19 pandemic. The first case was identified at the end of February 2020 and shortly thousands of patients overwhelmed the hospitals in the Northern provinces. Patients often needed hospital admission, with many of them requiring supplemental oxygen, mechanical ventilation, and Intensive Care Unit (ICU) kind of respiratory support.
When SARS-CoV-2 began to spread in our country, we supposed we were facing an explosion of cases of interstitial pneumonia similar, from a pathophysiological perspective, to those induced by influenza viruses, cytomegalovirus or Pneumocystis jirovecii among others. We all had in mind patients in respiratory distress, with rapidly deteriorating clinical conditions that would require a quick referral to the ICU, where protective invasive mechanical ventilation, pronation, and even Extra Corporeal Membrane Oxygenation (ECMO) could be provided.5
However, as soon as COVID-19 patients started to be admitted to our hospitals, we were all surprised to face completely different patients than expected. Most of them did not even complain of dyspnea. No sense of breathlessness, no rapid shallow breathing, no need for accessory muscles of respiration, despite devastating CT scan images or dramatically low PaO2/FiO2 ratios.
The response of these patients’ lungs to mechanical ventilation was also surprising. In the few cases we were forced to treat with Non Invasive Ventilation (NIV) as a bridge to intubation or for lack of other options, we found an unexpected respiratory response, as if the lungs were “soft”, instead of “stiff” as they are supposed to be in ARDS.
This disease confronted us with completely unanticipated clinical and pathophysiological behaviour, which confounded our convictions.
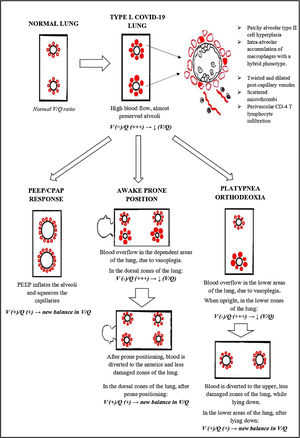
But it was just a matter of time before these mere impressions met science. In fact, in April 2020, Gattinoni et al. published a very interesting paper in Critical Care: “COVID-19 pneumonia: ARDS or not?”. In this article, the authors hypothesize the existence of two pathophysiological phenotypes of COVID-19 ARDS: the so called light phenotype (type L) and the heavy phenotype (type H).6
The light phenotype has preserved lung compliance (low elastance, i.e. high compliance), low ventilation/perfusion ratio (V/Q ratio), low weight and low reclutability. This phenotype is typical of the early phase of disease, but it can be seen in some severe cases as well.7 Gattinoni himself reported a cohort of 16 critically ill patients, with relatively normal lung compliance (50,2 ± 14,3 ml/cmH2O) despite a dramatically increased shunt fraction (0.5 ± 0,11). Such a wide discrepancy is unlikely in typical ARDS.8
The type H phenotype has high lung elastance (i.e. low compliance), high right to left shunt, high weight and high reclutability. This phenotype is often seen in the later phase of the disease. The patients with this phenotype are usually more severe and their condition clearly resembles classical ARDS. The histopathological features of the H phenotype, documented in autoptic series, are diffuse alveolar damage (DAD) with interstitial and alveolar proteinaceous edema, hyaline membranes, alveolar type II cells hyperplasia and, later on, myofibroblastic proliferation and collagen deposition, just like in ARDS. A significant quantity of macro- and micro thrombi have been reported in different autopsy series 9,10, pulmonary embolism being the main cause of death in around one third of cases in one study.9 Even if the significant increase of thrombotic events in DAD related to COVID-19 compared to other causes of DAD has been disputed 11, these findings suggest an important role of hypercoagulability in COVID-19, especially in the more severe disease. 9,10
Little is known of the L phenotype, which apparently has a unique pattern of behaviour, which is not akin to other known respiratory diseases.
Several scientific papers proposed interesting pathophysiological hypotheses and brilliant inferences, however only few studies documented what is actually happening in the lung.6,12–14 We believe that the histopathological features of early COVID-19, mirrored in HRTC scan findings15, could help explain the pathophysiological hallmark of the L Type of SARS-CoV-2 pneumonia.
What is happening down there in the lung?We examined the histopathologic and immune molecular features of 12 Covid-19 patients in the earlier stages of illness (<20 days from onset of symptoms). All these patients underwent transbronchial cryobiopsy. Morphological examination of these samples showed interesting characteristics, that were substantially different from the typical DAD.16
Lung tissue showed hyperplasia of interstitial capillaries. Postcapillary venules were twisted and dilated, showing thickened, edematous walls, without signs of vasculitis. We also recognized other signs of endothelial dysfunction, such as overexpression of PD-L1 and indoleamine 2,3 dioxygenase-1 (IDO-1), along with aggregates of platelets, whereas microthrombi were just an occasional finding.16. However, all our patients were treated at least with a prophylactic dose of heparin, possibly reducing this phenomenon.
Other peculiar features were patchy alveolar type II cell hyperplasia, perivascular CD4 T lymphocyte infiltration and intra alveolar accumulation of macrophages with a hybrid phenotype.16
In the study by Doglioni C et al.16 the research on expression of angiotensin converting enzyme 2 (ACE 2) receptor in lung tissue was also performed (data not published). However, ACE 2 appeared normally expressed in type 2 alveolar cells and endothelial cells.
As aforementioned, endothelial cells covering both venules and alveolar capillaries overexpressed IDO-1. This enzyme is involved in the regulation of vascular tone and remodeling. When overexpressed, it can exert a relevant role in maintaining the pulmonary venules relaxation and the capillary hyperplasia seen in our series.17 Moreover, typical findings of DAD were rare or absent. There were no hyaline membranes and foci of intra alveolar proteinaceous oedema were spotted only occasionally.16
The aim of this paper is to answer this crucial question: can the histopathological hallmarks of early COVID-19 explain the pathophysiology and clinical presentation of the L phenotype? In particular, we will focus on clinical features such as the so called “happy hypoxemia”, the response to awake prone positioning, the response to positive end expiration pressure (PEEP) or to continuous positive airways pressure (CPAP), and reports of patients with platypnea orthodeoxia.
Can histopathologal feature explain peculiar clinical presentations of l phenotype COVID-19?General pathophysiological considerationsAs mentioned earlier, our lung samples showed not atelectatic alveoli, with no hyaline membranes. Therefore, the alveolar side of the alveolar capillary barrier is almost preserved.
On the other hand, vascular structures appear to be substantially rearranged. The dilation and hyperplasia of alveolar capillaries, along with pulmonary venodilatation, can lead to blood overflow around these almost pristine alveoli.
Hence, the ventilation/perfusion ratio (V/Q) is reduced, since ventilation is preserved, whereas perfusion is increased. This could be the main reason for hypoxemia in the L phenotype of COVID-19 (Fig. 1).
Furthermore, alveoli appeared neither collapsed nor obliterated; therefore it is unlikely that atelectasis contribute significantly to COVID-19 respiratory failure.
The “dead space” effect seems to play a marginal role considering the almost complete absence of microthrombi in our lung samples, and the absence of pulmonary embolism of larger vessels on computed tomography pulmonary angiography (CTPA), as all patients underwent this exam before the cryobiopsy.16
This assumption is confirmed by Lang et al., as they showed, thanks to dual energy CT scan, hyper perfusion in the affected areas in the mild forms of SARS-CoV-2 interstitial pneumonia.18
The “happy hypoxemia”This is undoubtedly one of the most interesting clinical features of COVID-19 patients. Many patients present with pronounced arterial hypoxemia, yet without proportional signs of respiratory distress. In several cases, they do not even verbalize a sense of dyspnea. This phenomenon has been labeled as “happy dyspnea”.13
Tobin et al. presented 3 cases of extreme “happy hypoxemia”, with PaO2 ranging between 36 and 45 mmHg, in the absence of increased alveolar ventilation.19 Moreover, Guan reported dyspnea only in 18,7% of 1099 hospitalized COVID-19 patients, despite low PaO2/FiO2 ratios and abnormal CT scans.20
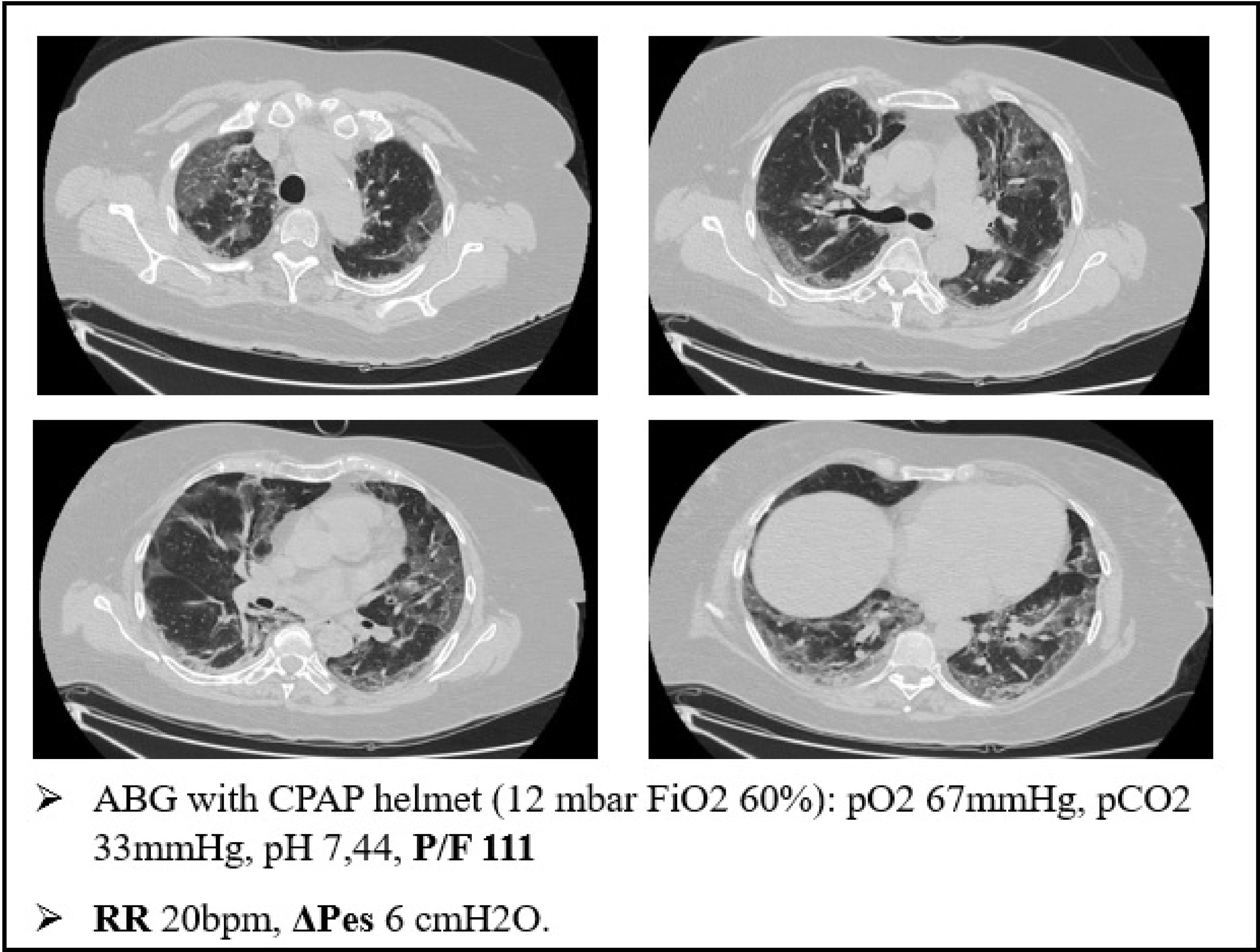
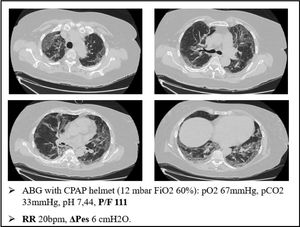
As an example of this phenomenon, we can also report one of our most plain cases of “happy hypoxemia”. Fig. 2 refers to a 67yo woman with no significant past medical history. The CT scan was taken 15 days after the onset of symptoms, and shows bilateral ground glass/crazy paving attenuation, with a visual severity score of 16/20. The patient developed severe hypoxemic respiratory failure and CPAP treatment was started. As displayed on Fig. 2, the patient was tachypneic and ABG during CPAP treatment showed severe respiratory failure. Tidal volume could not be recorded, but we did monitor the swing of esophageal pressure (ΔPes), thanks to a multifunctional nasogastric tube with a dedicated pressure transducer (NutriVent Sidam Group). The swing of esophageal pressure, during spontaneous breathing, reflects the respiratory effort.21 To reduce the confounding factor of breath to breath variability of the respiratory dynamic22, we measure esophageal pressure swing in at least two daily 3 min long recordings at rest and then calculate an average ΔPes. In this case, we measured a ΔPes of 6cmH2O: a normal respiratory effort, even if the patient had profound respiratory failure with tachypnea.
The causes behind this unusual phenomenon are yet to be fully understood. However, several hypotheses have been proposed.
First of all, dyspnea is generally defined as a sensation of difficult or labored breathing. It occurs when the demand for ventilation is out of proportion to the patient’s ability to respond. It is therefore different from tachypnea (rapid breathing) and hyperpnea (increased tidal ventilation).23,24
COVID-19 patients usually present with hypoxemia (low PaO2) and low PaCO2, caused by several confounding factors such as fever, hyperpnea due to hypoxemia, or anxiety associated to the arterial puncture itself.13
The respiratory chemosensors are highly sensitive to increasing level of PaCO2. Therefore, CO2 retention is one of the strongest stimuli to increase in respiratory drive and minute volume ventilation, contributing to dyspnea. On the other hand, hypoxemia alone plays a limited role in the sensation of breathlessness. Experimental models have shown that dyspnea only occurs when PaO2 drops below 40 mmHg, whereas when PaO2 ranges between 65 and 40 mmHg, the body responds with a rise in minute ventilation, increasing the respiratory rate, without dyspnea. Tachypnea and hyperpnea, not dyspnea, are therefore the clinical signs of impending hypoxemic respiratory failure.23,24
Tobin et al. reported a broad range in chemosensors’ sensitivity to PaCO2 and PaO2 in healthy subjects, and also in the same patient when the tests were performed at a different time of the day. Older age increases this variability as well, hence setting a precise threshold of chemosensitivity for the occurrence of dyspnea might be extremely complicated.22
Moreover, some comorbidities, such as diabetes mellitus (reported in roughly 16% of severe COVID-19 cases by Guan et al.20) might impair the perception of dyspnea.25 This phenomenon might therefore reduce the prevalence of dyspnea in diabetic COVID-19 patients, leading to a delay in seeking medical attention.
However, dyspnea is not just a matter of central chemosensors and PaCO2 sensitivity. In fact, dyspnea can also be caused by inputs from the mechanoreceptors in the respiratory tract and chest wall, and by fatigue or weakness of respiratory muscles due to altered lung and chest wall mechanics.24
The compliance of the respiratory system therefore plays a significant role in the genesis of dyspnea. As aforementioned, our lung biopsies show no signs of atelectasis or alveolar edema, leaving the lung with an almost preserved compliance. During the first days of infection, there is also no increase in airway resistance, thus the breathing effort remains low in many patients without preexisting lung disease when affected by COVID-19.13
This also suggests that dyspnea is a very important clinical sign of deterioration in COVID-19 patients. Dyspnea tells the clinicians that lung compliance is falling, and that the patient might be evolving from an L phenotype to a more life threatening H phenotype.13
The response to awake prone positioningProne invasive ventilation is a cornerstone of treatment of severe ARDS. It is a strategy to improve oxygenation when protective ventilation fails. In severe ARDS, prone positioning improves gas exchange by ameliorating the ventral dorsal transpulmonary pressure difference, reducing dorsal lung compression, and improving lung perfusion.26,27 It also reduces mortality, according to several papers.28
However, proning awake, spontaneously breathing patients with COVID-19 pneumonia is gaining acceptance. Initially, anecdotal evidence on the benefit of awake prone positioning in COVID-19 was reported, but now evidence is growing. This was one of the first surprises of COVID-19 patients, and it was very welcome, since it is an intervention with minimal risk and requiring minimum assistance.29–32
It is now clear that awake prone positioning leads to a quick improvement in arterial blood oxygenation in SARS-CoV-2 pneumonia. However, as our biopsies show no significant signs of parenchimal atelectasis nor alveolar edema, it seems unlikely that the beneficial effect of prone positioning in these patients is due to a better ventilation of the dorsal region of the lung.
The vascular changes seen in our histological samples may play a role in this phenomenon. As a matter of fact, recent reviews of CT scans highlight the importance of the vascular alterations in typical radiological findings of COVID-19. For example, Lang et al., using dual energy CT scan technology, described peculiar vascular enlargement and mosaic attenuation as a pattern of inefficient vasoregulation in the affected regions, besides the typical ground glass attenuation and consolidation. The authors labeled these features as “hyperemic halo” pattern.18
Moreover, in a case series of COVID-19 patients, Piciucchi et al. described a reversibility of venous dilation and changes in parenchimal density in CT scan images acquired after moving the patient from supine to prone position. Interestingly, in this series as soon as patients switch to the prone position, enlargement of pulmonary veins appears to diminish and changes in density of the ground glass and/or crazy paving areas are observed.15 These findings match with what we found in our biopsies. The typical ground glass attenuation/crazy paving of SARS-CoV-2 pneumonia could largely reflect the vascular changes taking place in the alveolar septa, instead of accumulation of proteinaceous edema or hyaline membranes in the alveolar spaces.
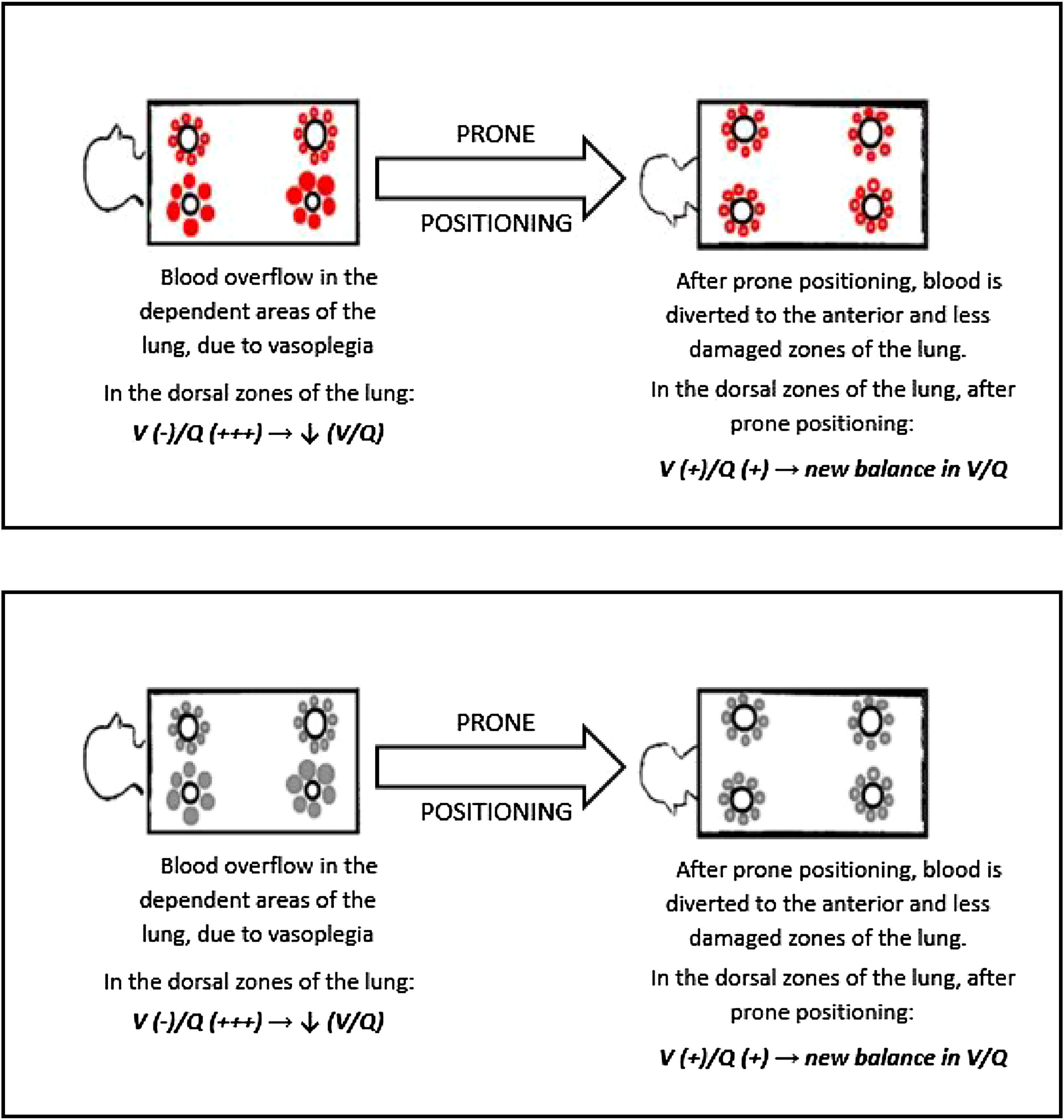
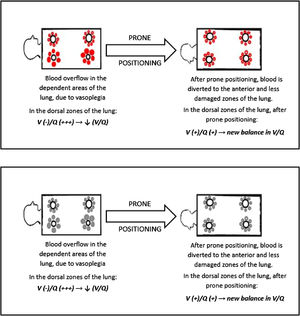
Our findings suggest that the injured areas of the lung are regions with high blood perfusion and rather normal alveoli, therefore in these areas the V/Q ratio reaches the lowest values. Prone positioning leads to a blood flow redistribution to the less damaged area, and hence to a new balance of the V/Q ratio, increasing arterial oxygen levels (Fig. 3)
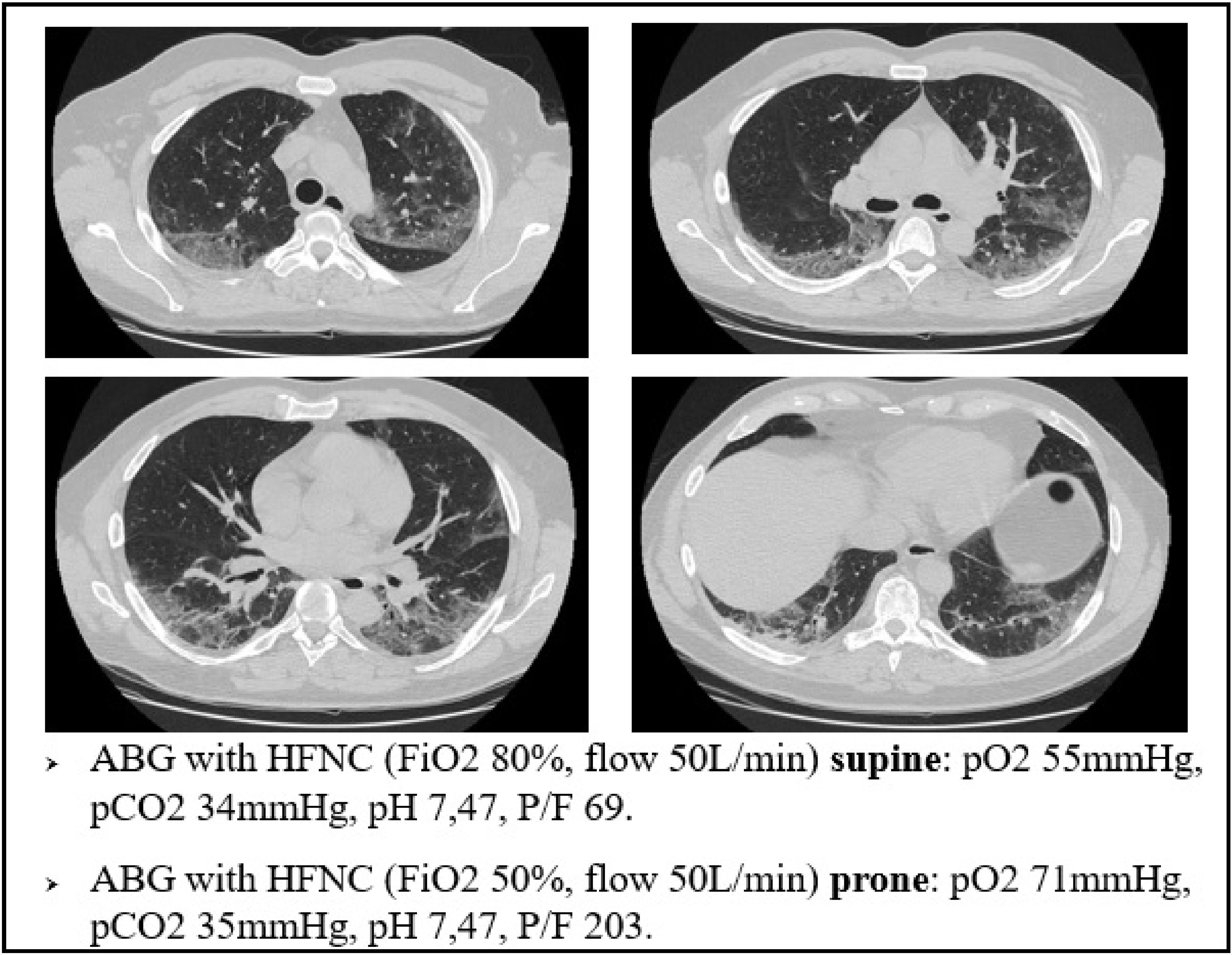
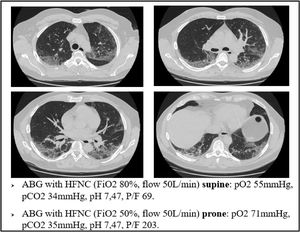
This mechanism is well represented in Fig. 4, a 49yo male with no past medical history. The CT scan was obtained after 9 days from the onset of symptoms, and displays the typical radiological findings of SARS-CoV-2 pneumonia, mainly in the dorsal region of the lungs. Visual severity score was 12/20. This was one of the patients that underwent lung biopsy. Lung tissue from the altered region of the right lower lobe showed areas of high blood flow, due to capillary hyperplasia and venous dilation, surrounding patent alveoli. Switching the patient to the prone position resulted in a rapid improvement in arterial blood oxygenation at ABGs.
Response to PEEP/CPAPCPAP is widely used in the COVID-19 pandemic, especially in patients treated outside the ICU. Patients treated with CPAP are usually affected by type L COVID-19.33–35
CPAP has been used for decades in a variety of patients to improve arterial blood oxygenation. The main role of CPAP is keeping the alveoli open: the so called “alveolar recruitment”. In fact, CPAP can push back the transudate from the alveolar space during acute cardiogenic edema, but it can also keep the lung open when facing exudate and atelectasis due to lung infections, inflammations or classical ARDS.36,37,5
However, how can we explain the striking role of CPAP in type L SARS-CoV-2 pneumonia, since our biopsies showed no sign of alveolar edema, hyaline membranes or atelectasis?
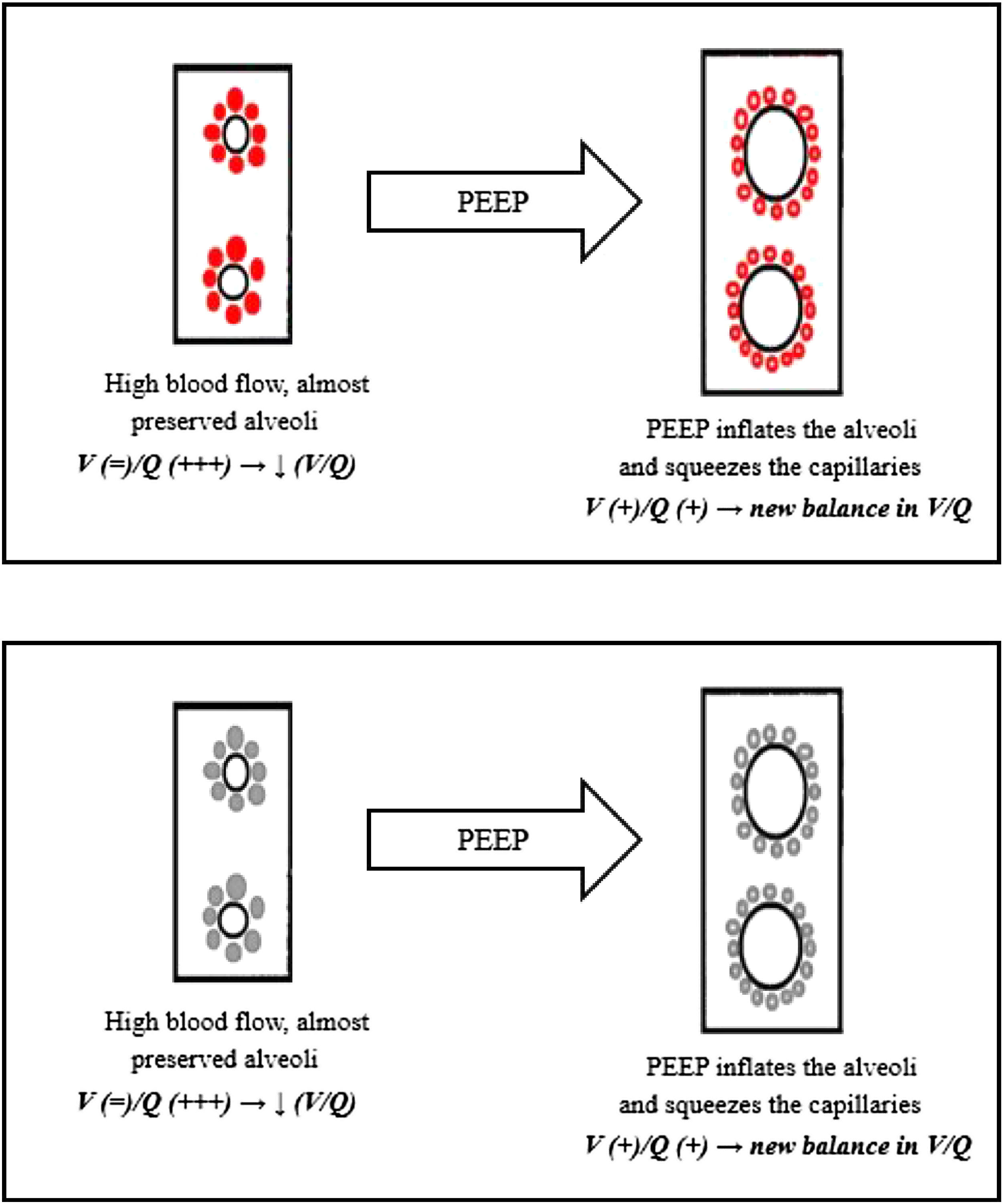
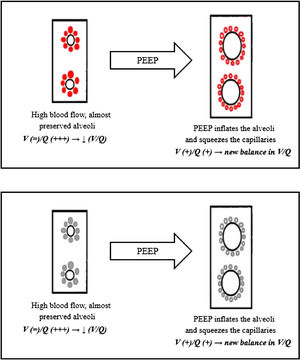
Again, we can explain this phenomenon as a matter of V/Q ratio. The increased alveolar pressure given by CPAP might help by inflating the alveoli, thereby squeezing the capillaries next to them, reducing the V/Q ratio inequality (Fig. 5)
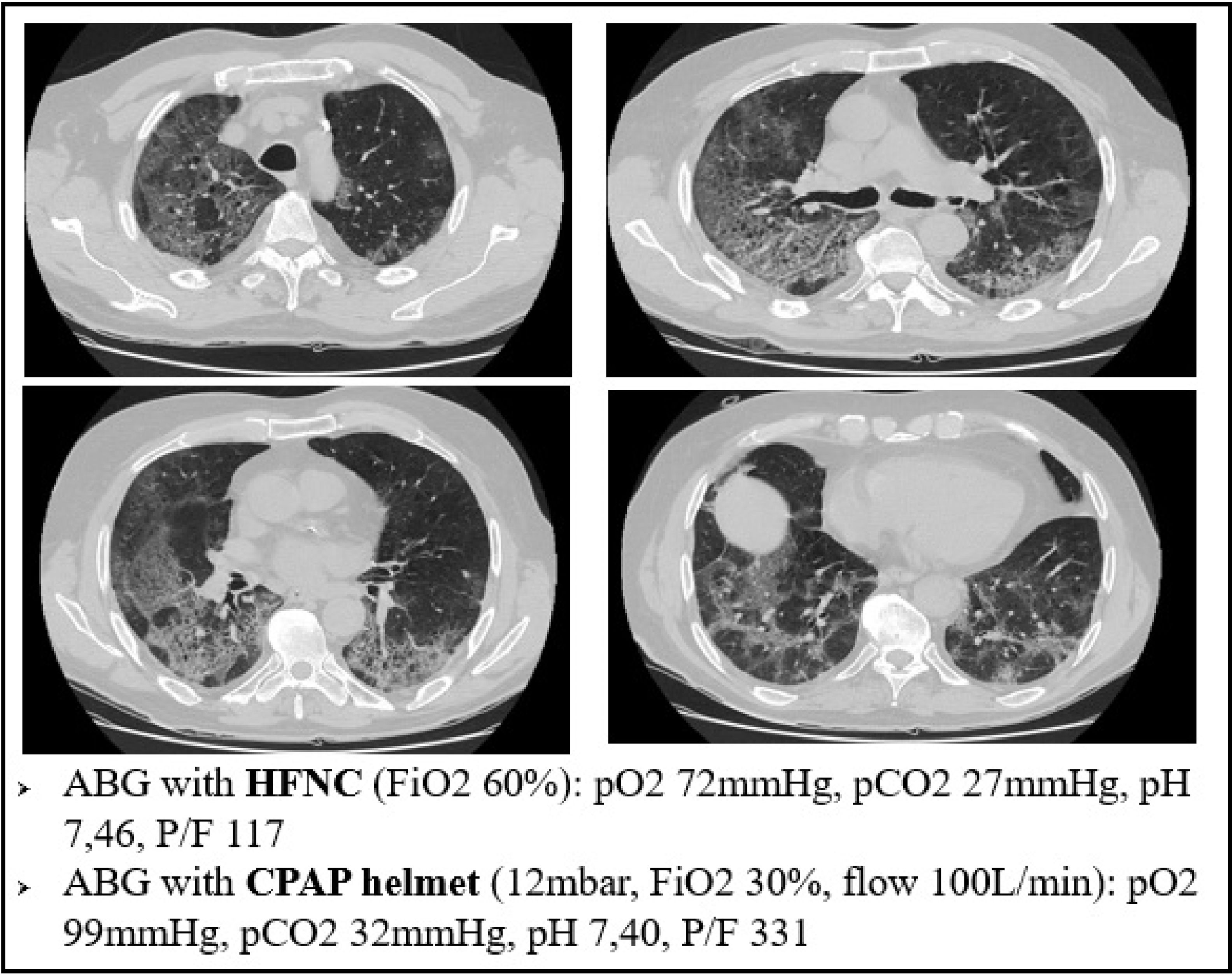
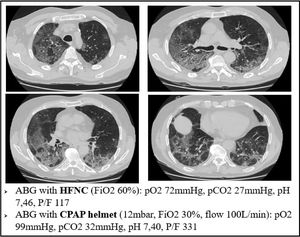
For example, Fig. 6 refers to a 76yo male, former smoker, with a past medical history of arterial blood hypertension and moderate chronic kidney disease. The CT scan was obtained after 7 days from the onset of symptoms and shows bilateral ground glass attenuation and crazy paving typical of the early phases of SARS-CoV-2 pneumonia. Visual severity score was 14/20. The high flow CPAP strongly ameliorated the arterial blood oxygenation after few minutes. The results of our biopsy suggest that such a quick improvement could be explained with a redistribution of lung perfusion, along with alveolar hyperinflation. A new balance in V/Q was reached.
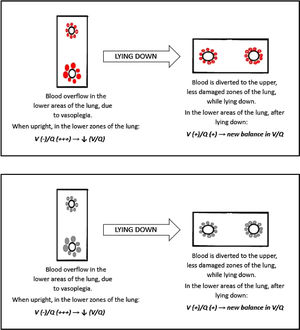
Platypnea OrthodeoxiaPlatypnea Orthodeoxia refers to an uncommon condition of positional dyspnea and hypoxemia, that occur when the patient is upright and are resolved with recumbency. This syndrome is suspected when normal arterial oxygen saturation (SpO2) is recorded while the patient is supine, followed by abrupt decline in saturations when upright.38
Apparently, this condition is less common in type L COVID-19 patients than the phenomena described before. Even so, some cases have been described, especially during recovery after the acute phase of SARS-CoV-2 pneumonia.39
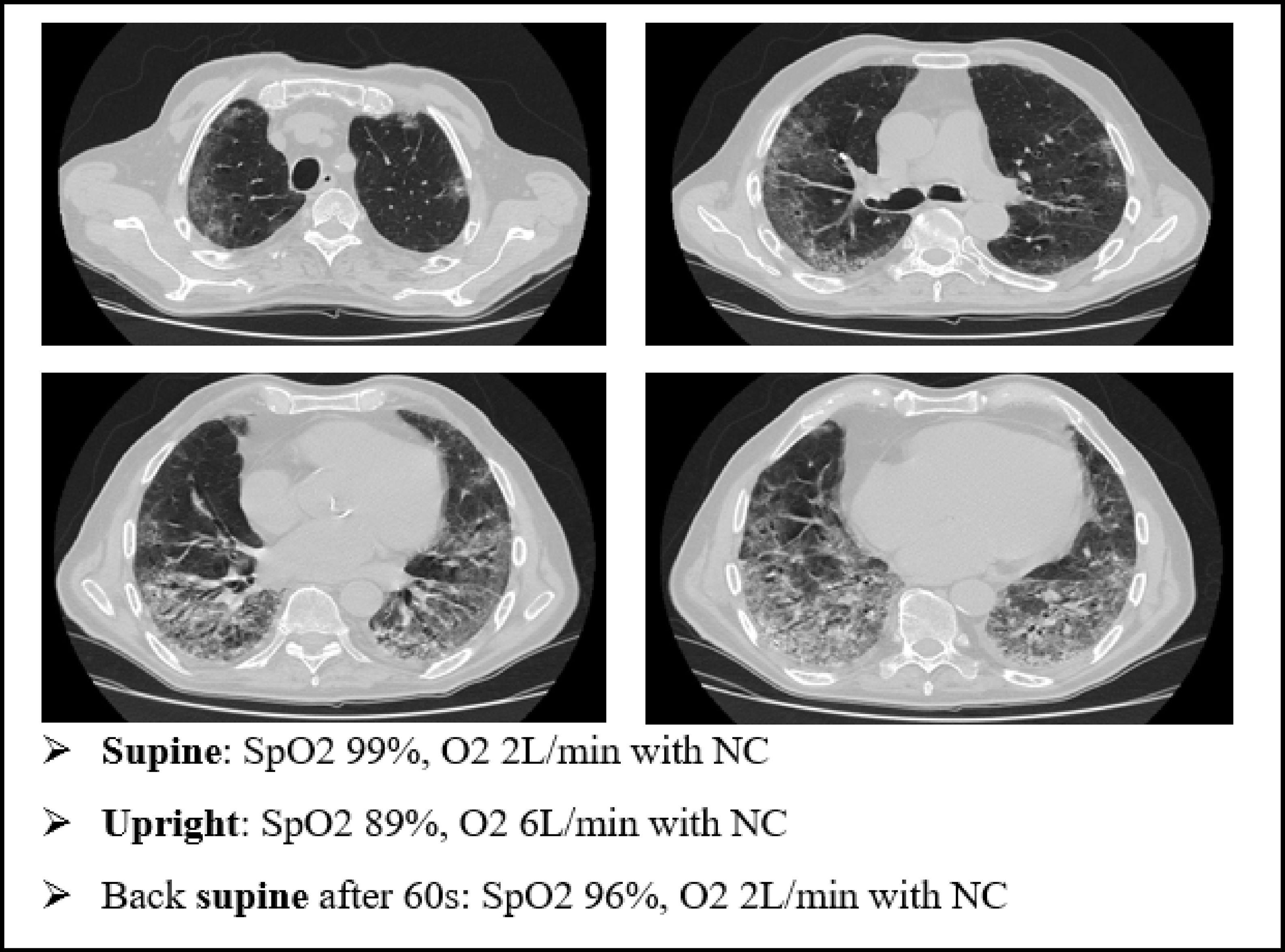
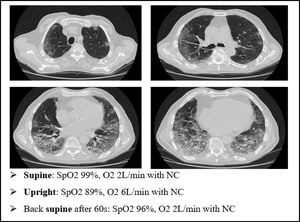
We present an explanatory case as seen in our ward (Fig. 7). This is an 80 yo male, former smoker, with a past medical history of arterial blood hypertension and benign prostatic hypertrophy. He was admitted in to our Semi intensive Respiratory Care Unit for SARS-CoV-2 pneumonia and treated with CPAP for several days. After the weaning from CPAP, the patient started to develop clinical signs of platypnea orthodeoxia. As shown in Fig. 4, he needed a relatively small amount of oxygen through nasal cannula (NC) to maintain a normal level of SpO2 while lying down. However, sitting up resulted in a drastic drop in SpO2 levels, requiring increase in O2 flow.
The patient underwent a CT scan. It was 18 days since the onset of symptoms. The CT scan showed bilateral ground glass attenuation, crazy paving and consolidation, especially in the lower lobes.
How can we explain this case of platypnea orthodeoxia, considering the CT scan and the information given by our biopsy?
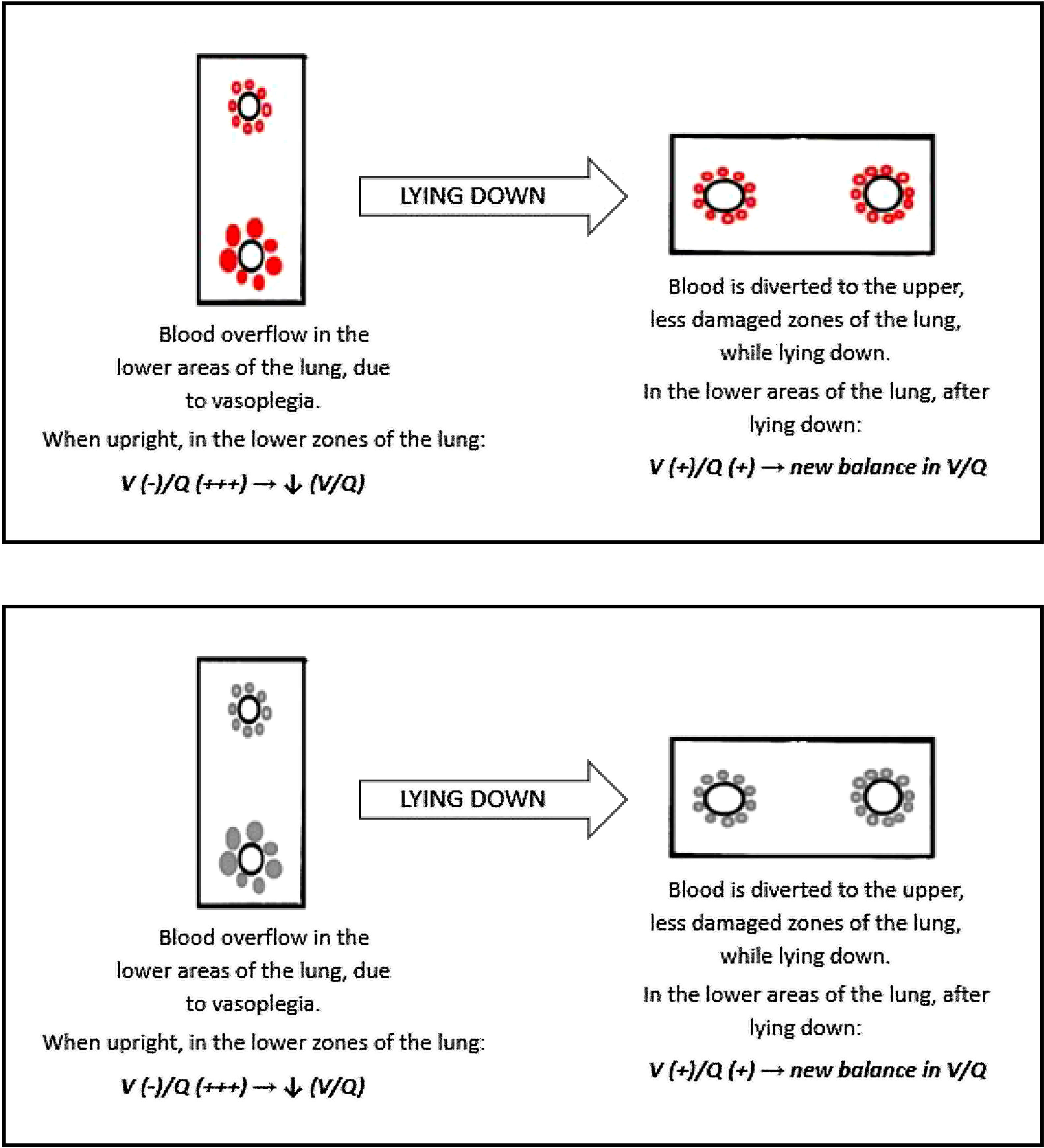
We could explain this phenomenon with a similar condition: the hepatopulmonary syndrome (HPS). Hepato-pulmonary syndrome is characterized by impairment in blood oxygenation due to intrapulmonary vascular dilations and shunting in the context of liver disease. HPS is defined as a syndrome characterized by a clinical triad: (1) advanced chronic liver disease, (2) arterial oxygenation defect, (3) widespread pulmonary vascular dilation. The pathogenesis of this condition is yet to be fully understood. These patients have extremely dilated pulmonary vasculature, with almost preserved ventilation, and hypoxemia is caused by a reduction of the normal V/Q ratio.40,41
Moreover, the vascular enlargement is usually more represented in the lower regions of the lungs, leading to a sudden increase in perfusion of the dependent areas of the lung as soon as the patient stands upright. This overflow causes further reduction in V/Q ratio, hence a swift worsening of hypoxemia in sitting position (Fig. 8).41
We can speculate a similar mechanism in type L COVID-19 patients showing platypnea orthodeoxia. The main histopathological feature of SARS-CoV-2 pneumonia is vascular enlargement, therefore if the COVID-19 lesions are more severe in the lower areas of the lungs (as in Fig. 7), it is possible for the patient to develop the platypnea orthodeoxia syndrome, with the same mechanism of HPS.
ConclusionsWe believe that the direct knowledge of the histopathological hallmarks of early COVID-19 could provide some plausible answers to the many questions that the L phenotype as hypothesized by Gattinoni L, et al. raises.6
We suggest that the pathophysiology of the L phenotype might be explained by the predominance of the shunt effect and V/Q ratio reduction due to: (1) the neoangiogenesis taking place in the interalveolar septa; (2) the “vasoplegia” due to the loss of muscular tone of the alveolar capillaries and pulmonary venules and veins; (3) the stability of the alveoli which do not display the typical damage seen in ARDS.
This could explain interesting clinical behaviour such as the CPAP response, the response to spontaneous breathing pronation and the platypnea orthodeoxia seen in some patients as summarized in Fig. 9.
Our hope is that these observations might contribute to identify more tailored respiratory support and to investigate a possible role of drugs affecting pulmonary vascular tone in the treatment of COVID-19 pneumonia.
Authors contributionsStefano Oldani and Claudia Ravaglia conceived the paper and equally contributed to write the draft down. Serena Bensai, Lara Bertolovic, Cristiano Colinelli, Sara Piciucchi, Corrado Ghirotti, Silvia Puglisi, Sabrina Martinello and Siro Simoncelli helped to collect data, to analyze them and to interpret of the described phenomena. Venerino Poletti contributed in the inception of the paper, reviewed it critically and supervised all the aspects of this paper.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not for profit sectors.
Ethical disclosureAll patients involved consented their clinical data to be used for this paper.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors would like to thank all the doctors, nurses, and personnel who fought, and keep fighting against the COVID-19 pandemic in our hospital. The authors would also like to thank the radiologists and pathologists who helped us better understand this disease.