STOP-BANG score (snore; tired; observed apnea; arterial pressure; body mass index; age; neck circumference and gender) can predict the risk of a patient having Obstructive Syndrome Apnea (OSA). The aim of this study was to evaluate the incidence STOP-BANG score≥3, in surgical patients admitted to the Post-Anesthesia Care Unit (PACU).
MethodsObservational, prospective study conducted in a post-anesthesia care unit (PACU) during three weeks (2011). The study population consisted of adult patients after noncardiac and non-neurological surgery. Patients were classified as high risk of OSA (HR-OSA) if STOP-BANG score≥3 and Low-risk of OSA (LR-OSA) if STOP-BANG score<3 (LR-OSA). Patient demographics, intraoperative and postoperative data were collected. Patient characteristics were compared using Mann–Whitney U-test, t-test for independent groups, and chi-square or Fisher's exact test.
ResultsA total of 357 patients were admitted to PACU; 340 met the inclusion criteria. 179 (52%) were considered HR-OSA. These patients were older, more likely to be masculine, had higher BMI, higher ASA physical status, higher incidence of ischemic heart disease, heart failure, hypertension, dyslipidemia and underwent more frequently insulin treatment for diabetes. These patients had more frequently mild/moderated hypoxia in the PACU (9% vs. 3%, p=0.012) and had a higher incidence of residual neuromuscular blockade (NMB) (20% vs. 16%, p=0.035). Patients with HR-OSA had a longer hospital stay.
ConclusionsPatients with HR-OSA had an important incidence among patients scheduled for surgery in our hospital. These patients had more co-morbidities and were more prone to post-operative complications.
A pontuação no STOP-BANG (Snore, Tired, Observed apnea, blood Pressure) pode prever o risco de um doente ter Síndrome da Apneia Obstrutiva Sono (SAOS). O objetivo deste estudo foi avaliar a incidência da pontuação STOP BANG> 3, em pacientes cirúrgicos internados na Unidade de Cuidados Pós-Anestésica (UCPA).
MétodosEstudo observacional e prospectivo conduzido numa UCPA, durante três semanas (2011). A população de estudo consistiu em doentes adultos após cirurgia não cardíaca e não neurológica. Os doentes foram considerados com alto risco de SAOS (AR-SAOS) se tinham um score de STOP-BANG ≥3 e de baixo risco de SAOS (BR-SAOS) se tinham score de STOP-BANG <3. Foram avaliados dados demográficos dos doentes e colhidas variáveis intraoperatórias e pós-operatórias. As características dos doentes foram comparadas através do teste de Mann-Whitney, teste t, qui-quadrado ou teste exato de Fisher.
ResultadosUm total de 357 doentes foram admitidos de UCPA e 340 preencheram os critérios de inclusão. Cento e setenta e nove (52%) tinham AR-SAOS. Estes doentes eram mais velhos, tinham maior probabilidade de serem do sexo masculino, tinham um Índice Massa Corporal superior, tiveram uma classificação maior no estado físico American Society Anesthesiologists, uma maior incidência de doença cardíaca isquémica, insuficiência cardíaca, hipertensão, dislipidemia e eram mais frequentemente doentes com diabetes em tratamento com insulina. Esses doentes tiveram mais frequentemente hipóxia leve/moderado na UCPA (9% versus 3%, P=0,012) e tiveram maior incidência de bloqueio neuromuscular residual (NMB) (20% versus 16%, P=0,035). Os doentes com AR-SAOS tiveram maior tempo de internamento hospitalar.
ConclusõesOs doentes com AR-SAOS tem uma alta incidência entre os doentes submetidos a intervenções cirúrgicas programadas no nosso hospital. Esses doentes tinham mais comorbilidades e foram mais propensos a ter complicações pós-operatórias.
Obstructive sleep apnea (OSA) is a common medical condition affecting 2–26% of the general population and it is the most prevalent breathing disturbance in sleep.1,2 OSA is characterized by repeated complete or partial collapse of the pharyngeal airway during sleep, causing cessation of airflow (apnoea) or shallow breathing (hypopnea). This pattern of sleep arousal, coupled with intermittent hypoxemia, is associated with serious adverse cardiovascular outcomes, including strokes, and leads to daytime somnolence and compromised neurocognitive function.3
The prevalence of OSA in the surgical population is higher than in the general population and it can vary widely according to the underlying medical condition.3 In particular, as many as 70% of patients undergoing bariatric surgery were found to have OSA.4 OSA has been recognized as a potential independent risk factor for adverse perioperative outcome.5 Compared to non-OSA patients, OSA patients undergoing surgical procedures are vulnerable to postoperative airway obstruction,6 myocardial ischemia congestive heart failure, stroke and oxygen desaturation.5,7,8
The gold standard for the diagnosis of OSA, polysomnography (PSG), is impracticable as a routine preoperative assessment tool for OSA because it is an expensive and labor intensive test.5 Many tools have been proposed for screening patients for OSA such as the Berlin questionnaire, the STOP questionnaire and the American Society of Anaesthesiologists (ASA) checklist – and the use of these tools improves the likelihood of identifying OSA preoperatively.3 The STOP questionnaire, showed a high sensitivity for detecting OSA (when score is ≥3): 93% and 100% for moderate and severe OSA, respectively; however, the specificity at the same cut-off is low: 47% and 37% for moderate and severe OSA, respectively, resulting in high false-positive rates. The STOP questionnaire is a scoring model consisting of 8 easily administrated questions starting with the acronym STOP-BANG (Appendix) and is scored based on Yes/No answers (scores 1/0).1
The aim of this study was to evaluate the incidence of patients with a STOP-BANG score≥3, in surgical patients admitted in the Post-Anesthesia Care Unit (PACU).
MethodsSubjects and settingsEthical approval for this study was provided by the Ethics Committee of Centro Hospitalar São João, Porto, Portugal. Written informed consent was obtained from all participants.
Centro Hospitalar São João, Porto, is an 1124-bed tertiary hospital in a major metropolitan area that serves 3,000,000 people. This prospective study was conducted in a 12-bed PACU over a 3-week period (from May 9th to May 27th, 2011). Every patient admitted to the PACU who was able to provide written informed consent was included in the study. Exclusion criteria were patient refusal, incapacity to provide informed consent, a score of <25 in the mini-mental state examination (MMSE), under 18 years of age, foreign nationality, known neuromuscular disease, urgent/emergent surgery and cardiac surgery, neurosurgery or other procedures that required therapeutic hypothermia.
All the patients were asked to complete the STOP questionnaire. Information concerning body mass index (BMI) age, neck circumference, and gender (Bang) were collected by a research assistant.
Patients were classified as being at high risk for obstructive sleep apnea (HR-OSA) if their STOP-BANG score was 3 or greater and as being at low risk of OSA (LR-OSA) if their score was less than 3.
The anesthesiologist in charge was blinded to patient involvement in the study. Anesthesia was provided and monitored according to the anesthesiologist in charge criteria, but this conduct followed minimum departmental standards. Neuromuscular blocking drugs (NMBD) were used for tracheal intubation, and additional boluses were provided, if needed. No written policy exists concerning the use of neuromuscular monitoring so this was performed at the discretion of the anesthesiologist. To ensure that the anesthesiologist remained blinded to the patients’ participation in the study, we did not attempt to observe the use or interpretation of TOF intraoperatively. The anesthesiologist was free to decide whether to reverse the neuromuscular blockade (NMB) with neostigmine at the conclusion of the surgical procedure.
Usually, the patient was extubated in the operating room and transferred to the PACU. Criteria for extubation included sustained head lift or hand grip for more than 5s, the ability to follow simple commands, a stable ventilatory pattern with acceptable arterial oxygen saturation (SpO2)>95%, and a TOF ratio greater than 0.80. All subjects were administered 100% oxygen by a facemask after tracheal extubation. The anesthesiologist was free to decide whether to administer oxygen during the time between transfer to the cart and admission to the PACU.
Upon arrival at the PACU all subjects were provided with oxygen either by a nasal cannula or face mask.
A standardized data collection sheet was completed for each patient. The recorded patient characteristics were: age, weight, height, body mass index (BMI), benzodiazepine administration before surgery, chronic benzodiazepine use, site of surgery (intra-abdominal, musculoskeletal or head and neck), American Society of Anesthesiologists physical status (ASA-PS), Revised Cardiac Risk Index (RCRI), drug or alcohol abuse, duration of preoperative fluid fasting, type of anesthesia, duration of surgery, use of nitrous oxide, adverse respiratory events in the PACU, postoperative pain level (VAS score), postoperative nausea and vomiting 6hours after surgery and 24h after surgery, length of stay in the PACU, and postoperative length of stay in hospital.
The surgical procedure was classified in terms of magnitude, as major (surgery in which body cavities or major vessels are exposed to ambient temperature such as major abdominal, thoracic, or major vascular, thoracic spine surgery with instrumentation, or hip arthroplasty), medium (surgery in which body cavities are exposed to a lesser degree such as appendectomy), and minor surgery (superficial surgery). Major surgery was defined as a surgery requiring a hospital stay of 2 or more days.
Clinical risk factors (a history of ischemic heart disease, history of compensated or prior heart failure, history of cerebrovascular disease, diabetes mellitus, and renal insufficiency) and surgical risk (high-risk defined as intrathoracic, intraperitoneal, or suprainguinal vascular surgery, or surgery involving large blood loss or fluid shifts) were defined according to the Cardiac Risk Stratification for Noncardiac Surgical Procedures of the 2007 guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines.9
Data for other preoperative clinical information regarding chronic obstructive pulmonary disease (COPD), hypertension and dyslipidemia were collected from routine clinical documentation entered into the institution's perioperative clinical information system.
Residual neuromuscular blocking was defined as a TOF<0.9 and it was quantified at admission to the PACU using acceleromyography of the adductor pollicis muscle (TOF-Watch®).10,11
The patients were screened for delirium at PACU discharge and in the ward the next day after surgery using the nursing delirium screening scale (Nu-DESC).12 Patients with a Nu-DESC score of 2 or more points during at least at 1 evaluation were considered delirium positive. Patients were tested for delirium by the research team at the time they were formally declared to be ‘ready for discharge’ to the regular ward by the physician in charge of the recovery room. In addition, the patients were seen on the morning of the first postoperative day.
Postoperative respiratory complicationsEach postoperative ARE was defined on the data collection sheet using the following criteria according to the classification described by Murphy et al13:
- 1.
Upper airway obstruction requiring an intervention (jaw thrust, or oral or nasal airway);
- 2.
Mild-moderate hypoxia (SpO2 of 93–90%) on 3L nasal cannula O2 that was not improved after active interventions (increasing O2 flows to >3L/min, application of high-flow face mask O2, verbal requests to breathe deeply and tactile stimulation);
- 3.
Severe hypoxia (SpO2<90%) on 3L nasal cannula O2 that was not improved after active interventions (increasing O2 flows to >3L/min, application of high-flow facemask O2, verbal requests to breathe deeply, and tactile stimulation);
- 4.
Signs of respiratory distress or impending ventilatory failure (respiratory rate >20 breaths per minute, accessory muscle use, and tracheal tug);
- 5.
Inability to breathe deeply when requested to by the PACU nurse;
- 6.
Patient complaining of symptoms of respiratory or upper airway muscle weakness (difficulty breathing, swallowing, or speaking);
- 7.
Patient requiring reintubation in the PACU;
- 8.
Clinical evidence or suspicion of pulmonary aspiration after tracheal extubation (gastric contents observed in the oropharynx and hypoxemia).
The PACU nurses observed the patients continuously during the PACU stay and contacted a study investigator immediately if an ARE was observed. The inability to breathe deeply and the assessment of symptoms of respiratory or upper airway muscle weakness were assessed at 10-minute intervals. A study investigator examined the patient to confirm that the patient met at least one of the criteria for an ARE.
Statistical methodDescriptive analysis of variables was used to summarize data. Ordinal and continuous data found not to follow a normal distribution, based on the Kolmogorov–Smirnov test for normality of the underlying population, are presented as median and interquartile range. Normally distributed data are presented as mean and standard deviation (SD).
A univariate analysis was performed to identify differences between HR-OSA and LR-OSA patients by using the Mann–Whitney U-test to compare continuous variables and the Chi-square or Fisher's exact test to compare proportions between the 2 groups of subjects. Differences were considered statistically significant when P was <0.05.
A univariate analysis was performed to identify predictors for ARE.
Multiple regression binary logistic was used with forward conditional method in the model in order to identify independent predictors for ARE. In this model, all covariates with p<0.05 in the univariate analyses were entered and an odds ratio (OR) and 95% Confidence Interval (95% CI) were calculated.
Data were analyzed using SPSS software for Windows Version 19.0 (SPSS Inc., Chicago, IL, USA).
ResultsFrom the 357 patients consecutively admitted to the PACU during the study period, a total of 340 patients were studied. Seventeen patients were excluded: 7 patients were admitted to a surgical intensive care unit, 3 patients were unable to provide informed consent or had a MMSE <25, 3 patients did not undergo surgery, 1 patient underwent neurosurgery, 1 patient was less than 18 years old, 1 patient did not speak Portuguese and 1 patient refused to participate.
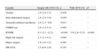
Of the 340 patients included in the analysis (Table 1), 179 (52%) were classified as HR-OSA. These patients were older (mean age of 61.7±13.4 vs. 48.8±16.0 years and median age 63 vs. 47 years, p<0.001), were more likely to be men (63% vs. 21%, p<0.001), had higher BMI (mean 29.1±6.0 vs. 25.1±4.4kg/m2, median 28 vs. 24kg/m2, p<0.001) higher ASA physical status (ASA III, IV or V 26% vs. 10%, p<0.001) and higher RCRI scores (RCRI>2 in 7% vs. 2%). Patients with HR-OSA had a higher incidence of ischemic heart disease (10% vs. 2%, p=0.001), heart failure (8% vs. 3%, p=0.032), hypertension (62% vs. 19%, p<0.001), dyslipidemia (41% vs. 12%, p<0.001) and underwent more frequently insulin treatment for diabetes (24% vs. 4%, p<0.001). The prevalence of cerebrovascular disease (4% vs. 1%, p=0.116) and preoperative serum creatinine >2mg/dl (6% vs. 4%, p=0.394) were not significantly different between the 2 groups.
Patient baseline characteristics (n=340).
| Variable | HR-OSA (n=179) | LR-OSA n=161 | p |
| Age, median (IQR) | 63 (55–71) | 47 (36–61) | <0.001c |
| Gender, n (%) | <0.001a | ||
| Male | 113 (63) | 33 (21) | |
| Female | 66 (37) | 128 (80) | |
| Site of Surgery, n (%) | |||
| Intraabdominal | 76 (43) | 47 (29) | 0.011a |
| Musculoskeletal | 88 (49) | 91 (57) | 0.175a |
| Head and Neck | 15 (8) | 23 (14) | 0.084a |
| Body mass index (kg/m2), median (IQR) | 28 (25–32) | 24 (22–28) | <0.001c |
| ASA physical status, n (%) | <0.001a | ||
| I/II | 132 (74) | 145 (90) | |
| III/IV/V, n (%) | 47 (26) | 16 (10) | |
| High risk surgery, n (%) | 57 (32) | 36 (22) | 0.050a |
| Ischemic heart disease, n (%) | 18 (10) | 3 (2) | 0.001b |
| Congestive heart disease, n (%) | 15 (8) | 5 (3) | 0.032b |
| Cerebrovascular disease, n (%) | 7 (4) | 2 (1) | 0.116b |
| Insulin therapy for diabetes, n (%) | 42 (24) | 7 (4) | <0.001a |
| Renal insufficiency, n (%) | 10 (6) | 7 (4) | 0.394a |
| RCRI, n (%) | 0.030b | ||
| RCRI≤2 | 167(93) | 158 (98) | |
| RCRI>2 | 12 (7) | 3 (2) | |
| Hypertension, n (%) | 111(62) | 30 (19) | <0.001a |
| Dyslipidemia, n (%) | 73(41) | 19 (12) | <0.001a |
| COPD, n (%) | 15 (8) | 4 (3) | 0.015b |
| Type of anesthesia, n (%) | 0.009a | ||
| Loco regional | 46 (26) | 23 (14) | |
| General/combined | 133 (74) | 138 (86) | |
| Magnitude of surgery, n (%) | |||
| Minor | 14 (8) | 21 (13) | 0.114a |
| Medium | 108 (63) | 104 (65) | 0.481a |
| Major | 57 (32) | 36 (22) | 0.033a |
| Bariatric surgery, n (%) | 12 (7) | 2 (1) | 0.016b |
| Duration surgery, median (IQR) | 87 (50–135) | 75 (50–120) | 0.173c |
| Post-operative delirium hospital, n (%) | 23 (13) | 16 (10) | 0.400a |
| Temperature on admission, median (IQR) | 35.3 (34.9–35.7) | 35.4 (34.9–35.7) | 0.158c |
| <35°C, n (%) | 6 (25) | 104 (33) | 0.424a |
| Length of PACU stay minutes, median (IQR) | 96 (67–145) | 91 (65–120) | 0.064c |
| Length of Hospital stay (days), median (IQR) | 5 (2–8) | 3 (2–6) | 0.010c |
| Perioperative muscle relaxants use, n(%) | 99 (55) | 106 (66) | 0.048a |
| Neuromuscular residual blocking, n (%) | 35(20) | 25 (16) | 0.035a |
HR-OSA patients were more likely to undergo intra-abdominal surgery (43% vs. 29%, p=0.011) and surgery of major magnitude (32% vs. 22%, p=0.033), had more frequently loco regional anesthesia as a unique technique (26% vs. 14%, p=0.009) and less frequently had general or combined general and loco regional anesthesia (74% vs. 86%, p=0.009). Patients classified as HR-OSA had a higher incidence of residual NMB (RNMB) (20% vs. 16%, p=0.035).
In relation to respiratory events (Table 2) that occurred in the PACU, HR-OSA patients had more frequently experienced mild/moderated hypoxia (9% vs. 3%, p=0.012). The length of the hospital stay (median 5 days vs. 3 days, p=0.01), but not the length of the PACU stay (96 vs. 91minutes, p=0.064), was higher in these patients.
Respiratory complications (n=340).
| Variable | All (n=340) | HR-OSA n=179 | LR-OSA n=161 | p |
| Respiratory events | 64 (19) | 39 (22) | 25 (16) | 0.140 |
| Obstruction airway | 3 (1) | 1 (1) | 2 (1) | 0.475 |
| Hypoxia (mild/moderate) | 19 (6) | 15 (9) | 4 (3) | 0.012 |
| Hypoxia Severe | 5 (2) | 3 (2) | 2(1) | 0.529 |
| Respiratory failure | 7 (2) | 5 (3) | 2(1) | 0.253 |
| Inspiratory capacity decreased | 49(15) | 28 (17) | 21 (13) | 0.407 |
| Muscle weakness | 11 (3) | 8 (5) | 3 (2) | 0.132 |
In multivariate analyses for ARE occurrence (Table 3) and after adjustment for univariate predictors (gender, intra-abdominal surgery, major surgery, cardiovascular high-risk surgery, general anesthesia and/or combined anesthesia, use of neuromuscular relaxants and postoperative residual neuromuscular blockade) the occurrence of residual neuromuscular blockade was identified as an independent predictor of ARE.
Multivariate regression analysis for predictors of adverse respiratory events.
| Variable | Simple OR (95% CI) | p | bOR (95% CI) | pa |
| Gender | 1.9 (1.0–3.3) | 0.038 | – | |
| Intra-abdominal surgery | 1.8 (1.0–3.0) | 0.049 | – | |
| General/combined anesthesia | 2.9 (1.3– 6.6) | 0.013 | – | – |
| NMBD use | 2.8 (1.5–5.3) | 0.002 | – | – |
| RNMB | 6.1 (3.1 –12.2) | <0.001 | 5.9 (2.8–12.5) | <0.001 |
| High risk surgery | 2.3 (1.3–4.1) | 0.004 | – | – |
| Major surgery | 1.8 (1.0–3.2) | 0.045 | – | – |
| HR-OSA | 1.5 (0.9–2.6) | 0.145 | – | – |
In these analyses HR-OSA was not a determinant for ARE (p=0.142) and was not entered in the multivariate model. HR-OSA is shown in Table 3 but it was not introduced in the model because it was not considered determinant for ARE.
DiscussionThe incidence of patients with HR-OSA was 52% in our study, which indicates that this is an important matter for concern among patients scheduled for surgery in our hospital.
We relied on a STOP-BANG score≥3 for this study, since this was demonstrated to be a highly sensitive test for moderate/severe OSA. Indeed, Chung et al.14 suggested that this cut-off is appropriate for the surgical population14 and further stated that the STOP-BANG questionnaire is concise, easy to use, and its importance lies in the identification of patients at high risk of OSA which may help to prevent postoperative complications.
The HR-OSA group of patients had a significantly higher percentage of men and as expected the BMI was significantly different between the LR-OSA and HR-OSA groups (p<0.001). Similar results have been reported previously.15,16 The importance of these findings is revealed by the presence of these variables in different tools such as the sleep apnea clinical score (SACS) and even the STOP-BANG, used to assess OSA severity.
The association between increased morbidity and untreated OSA is well established, which may result in an increased mortality rate.17,18 Furthermore, these patients are at a greater risk of developing cardiovascular disease. Indeed, HR-OSA patients had more frequently cardiac comorbidities (ischemic heart disease and congestive heart failure), hypertension, dyslipidaemia and pulmonary chronic disease in our study. These comorbidities may, however, have reflected higher ASA and RCRI scores, which would indirectly indicate the presence of more important associated comorbidities in patients at a higher risk of having OSA.
Our results show that HR-OSA patients more frequently underwent bariatric surgery, abdominal surgery, and major surgery. Thus, it is possible that the risks associated with major surgery and bariatric surgery may account for the higher complication rates rather than the diagnosis of HR-OSA in itself.
We found that the length of the hospital stay was higher in HR-OSA patients, which is in agreement with the study of Liu et al.19 This suggests postoperative hypoxemia is associated with adverse outcomes including the need for more frequent respiratory interventions and more intensive monitoring which may led to a longer hospital stay
The incidence of RNMB was higher in HR-OSA patients. In addition, a higher incidence of RNMB was found in patients with a high BMI,20,21 which may be due to the accumulation of neuromuscular blocker in the adipose tissue. These data suggest that enhanced monitoring is important when HR-OSA patients are exposed to additional risk factors for respiratory complications like RNMB, or when these patients are given drugs that enhance neuromuscular blockade.
Perioperative complications, including respiratory events, were more frequent in patients with HR-OSA. Compared with patients without this diagnosis, we found that the HR-OSA patients more frequently experienced mild/moderated hypoxia in the PACU and there was a greater risk of postoperative hypoxemia in HR-OSA patients in comparison with those without the diagnosis. This is consistent with the results of the study of Gali et al.,15 which found that respiratory events were more frequent in patients with a high risk of OSA. However, in our study, as in the study of Chung et al.22 analysis of perioperative adverse events did not show significant respiratory morbidity in HR-OSA patients compared to the LR-OSA patients. In fact in our study we did not find that HR-OSA was a determinant for ARE.
This study has several limitations. The first and most important limitation is that it was not possible to compare the results of the STOP-BANG questionnaire with a definitive polysomnographic diagnosis.
Second, our patients may have been at high risk for postoperative events without having OSA. The use of the STOP-BANG score is as an instrument to predict the risk of a patient to having OSA may have led to a high incidence of false positive results. It is possible that a higher score should have been used because recent studies have shown that the STOP-BANG score has a higher accuracy in detecting moderate to severe OSA patients in a surgical population when the score is greater than or equal to 5.1
Third, the respiratory events were only registered in the PACU and complications that could have occurred after PACU discharge were not considered. This might be viewed as a major limitation since respiratory complications after surgery may be associated with adverse outcomes that occur during the hospital stay after PACU discharge.
Fourth, the fact that HR-OSA patients more frequently underwent major surgery may be viewed as a confounding factor and a limitation of the study because it indicates that the patient groups were non-homogeneous.
In conclusion, the principal findings of this study are as follows:
- -
HR-OSA patients have a higher incidence of postoperative respiratory complications;
- -
mild/moderate hypoxia was the most frequent pulmonary adverse event in the immediate postoperative period that occurred more frequently in HR-OSA patients;
- -
HR-OSA patients had more comorbidities including ischemic heart disease, congestive heart failure, diabetes treated with insulin, chronic pulmonary disease, hypertension and dyslipidemia;
- -
HR-OSA patients required a longer stay in the hospital.
- -
HR-OSA was not a determinant for ARE.
The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.