Pemetrexed is a chemotherapeutic agent that inhibits three enzymes involved in the folate pathway, all of which are involved in nucleotide synthesis.1 Nowadays, it is widely used in advanced non-squamous non-small cell lung cancer (NSCLC): as first-line in combination with platinum; as maintenance monotherapy in non-progression patients after first-line chemotherapy with platinum based combination; and as second-line monotherapy.2 It is also the standard of care in advanced malignant pleural mesothelioma (MPM).2 Pemetrexed is known for its modest adverse effects despite the fact some pemetrexed-induced interstitial lung diseases (ILD) have been reported namely acute interstitial pneumonia (AIP), acute eosinophilic pneumonia or non-specific interstitial pneumonia (NSIP).3,4
The authors present the case of a 57 years old man, diagnosed with a stage IIIB (cT4N2M0) pulmonary adenocarcinoma. Molecular analyzes showed no epidermal growth factor receptor (EGFR) mutations or anaplastic lymphoma kinase (ALK) rearrangements. He was treated with concurrent chemotherapy (carboplatinum and vinorelbine) and radiotherapy, ceasing treatment with partial remission.
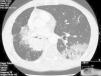
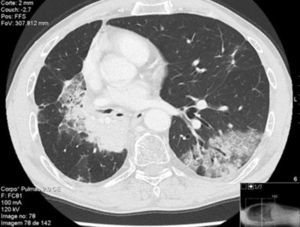
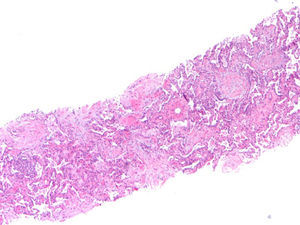
Thirteen months after finishing first-line therapy progression with multiple contralateral nodules was observed and histologically confirmed. At this point, treatment was proposed with pemetrexed 500mg/m2 combined with carboplatinum regimen every 21 days. Supportive medication with intramuscular vitamin B12 9/9w and folic acid 5mg per os every day was added. One week after second administration of chemotherapy, a CT scan evidenced new ground-glass opacity in the left lower lobe (Fig. 1). Patient was only presenting slight cough. He had no fever or respiratory distress signs. Primary differential diagnoses were chemotherapy-induced ILD versus pulmonary infection or disease progression. At that moment it was decided to discontinue chemotherapy. No bacterial pathogen was cultured in sputum. CT-guided transthoracic core needle biopsy (18G) revealed fairly preserved lung architecture, with fibroblast proliferation in polypoid configuration, slight lymphoplasmacytic inflammatory infiltrate in the interstitium and some macrophages with xanthomatous cytoplasm in the alveolar spaces (Fig. 2), compatible with organizing pneumonia (OP). The patient was treated with oral prednisolone 60mg/day (1mg/kg/day) for 2 weeks, 40mg a week and a subsequent decrease of 10mg per week. One month after starting steroid therapy chest-CT evidenced almost complete resolution of ground-glass opacity. At the present moment it is proposed that the patient receive immunotherapy with nivolumab, following his total recovery from drug-induced ILD and after reduction of prednisolone to 10mg/day or less.
Some chemotherapeutic agents have been associated with pulmonary toxicity and pemetrexed is one of them. Although the mechanism of injury is unclear, it may be similar to methotrexate. Toxicity can result from a hypersensitivity reaction or a direct toxic injury to alveolar epithelial walls.1 A large study reported it as a rare event with incidences of pemetrexed-induced ILD in MPM and NSCLC of 1.1% and 1.8%, respectively. Some risk factors have been described for such an outcome: male gender, age above 60 years, pre-existing ILD and smoking history.4 In our report, the patient presented two of the described risk factors – male gender and smoking history.
Drug-induced ILD is a diagnosis of exclusion based on clinical manifestations, imagiological patterns, lung histology and resolution of abnormalities after treatment discontinuation.1 In our report, the patient has previously been exposed to carboplatinum with no significant side effects, so an OP secondary to pemetrexed was postulated as the most probable diagnosis. Ochiai et al. reported radiation-induced OP until 18 months after exposure to radiotherapy. This has to be considered as another possible cause of OP. These lung lesions occurred outside the radiation field however they had a strong association with a prior history of symptomatic radiation pneumonitis (RP).5,6 In our case, previous RP was not found.
The potential role of other drugs administered in this chemotherapy regimen or radiotherapy per se or in combination with pemetrexed, could be the cause of the observed ILD. Rechallenge with pemetrexed in order to definitely confirm its causality would be dangerous and unethical.
To the best of our knowledge, OP secondary to pemetrexed has not been reported yet. Tomii et al. observed no specific parenchymal abnormality, yet the majority of cases referred to AIP and diffuse ground-glass opacity pattern. In the case of presentation as AIP or NSIP, the clinical course has been serious and treatment with intravenous steroids and pemetrexed discontinuation has been the main choice.4 Nevertheless, mortality rate was around 46%.1,4 In our report, OP appeared after second course of pemetrexed treatment. Clinical presentation was just slight symptoms with an almost complete imagiologic recovery in one month. This is in line with the favorable prognosis and good steroid responses described for OP. Although it is usual to follow prolonged tapering corticosteroid scheme in OP, a short scheme was chosen taking into account that the probable etiological agent was suspended and the need for early introduction of immunotherapy.
A recent report described a nivolumab-induced OP in a melanoma patient.7 Even though it was proposed to initiate nivolumab as it is recommended as second-line in NSCLC, particularly when there is high tumor expression of PD-L1 (60% in this case). These patients are often challenging not only because of the difficulty controlling the tumor but also due to the need to know how to manage the side effects of the therapy itself.
In the literature, drug-induced OP has sometimes been described for other chemotherapeutic agents. However, most publications do not specify the type of pemetrexed-induced ILD described. Therefore some of the cases described as “pneumonitis” may have been OP insufficiently characterized. In clinical practice they are often misdiagnosed as progressive diseases or pulmonary infections. Lack of recognition of these side effects will lead to unnecessary changes in therapeutic regimens which could sometimes be manageable without withdrawal of the drug. To sum up, our report emphasizes the importance of clarifying the true nature of these imagiological findings in order to ensure correct diagnoses and not subject patients to unnecessary and harmful treatments.
Conflicts of interestThe authors have no conflicts of interest to declare.
Author contributionsNuno Pereira and Sara Conde conceived the idea. Nuno Pereira, Sérgio Campainha and Ana Barroso collected the data and wrote the manuscript. Antónia Furtado was responsible for cytopathologic analysis. All the authors have read and approved the final version.