Advances in the management of patients with neuromuscular diseases (NMDs) have improved patient survival1–3 with increasing pregnancies prevalence.4 NMDs have a broad spectrum of presentation and a subgroup of these women are at risk of developing pulmonary complications (PCs) mainly due to respiratory muscle weakness leading to hypoventilation and ineffective cough.4–8 During pregnancy the growing fetus further impairs diaphragm excursion and increases respiratory muscles load, rising the risk of alveolar hypoventilation, ineffective cough and PCs mainly in the third trimester and at the time of delivery.4,9–12 In addition, cesarean section may be often required in these women, due to abdominal and truncal muscle weakness commonly seen even in mild disease.13 As a consequence, pregnancy in patients with NMDs may be associated with a significant risk of morbidity.8,14 Noninvasive ventilation (NIV) used in combination with mechanical insufflation-exsufflation (MI-E) can successfully treat hypoventilation and airway secretion retention in patients with NMDs, thereby preventing PCs, prolonged intubation, and tracheostomy.14–18
Our review of the literature found only isolated case reports of pregnant patients with severe chronic ventilatory failure due to NMDs such as polio, spinal muscular atrophy, limb-girdle muscular dystrophy, amyotrophic lateral sclerosis and mitochondrial myopathies.10,12,13,18–24 These case reports showed that different NMDs may present similar respiratory impairment such as hypoventilation and airway secretion retention. They also claimed that peri‑partum use of NIV may prevent PCs, allowing successful pregnancy and the delivery of healthy neonates despite severe baseline disability.
We hypothesized that NMDs pregnant women may benefit from a protocol for identifying women with pulmonary risk factors and preventing, in this subgroup, PCs by applying NIV combined with MI-E in the peri‑partum period
To test our hypothesis, we designed a multicenter protocol (protocol No. 473, November 4th, 2015, approved by our Institutional Review Board (IRB) with code n.175246/AR) enrolling consecutive pregnant women with NMDs undergoing cesarean section or spontaneous labor in a network of seven Italian hospitals (IT-NEUMA-Pregn study) to identify patients at risk of developing PCs.
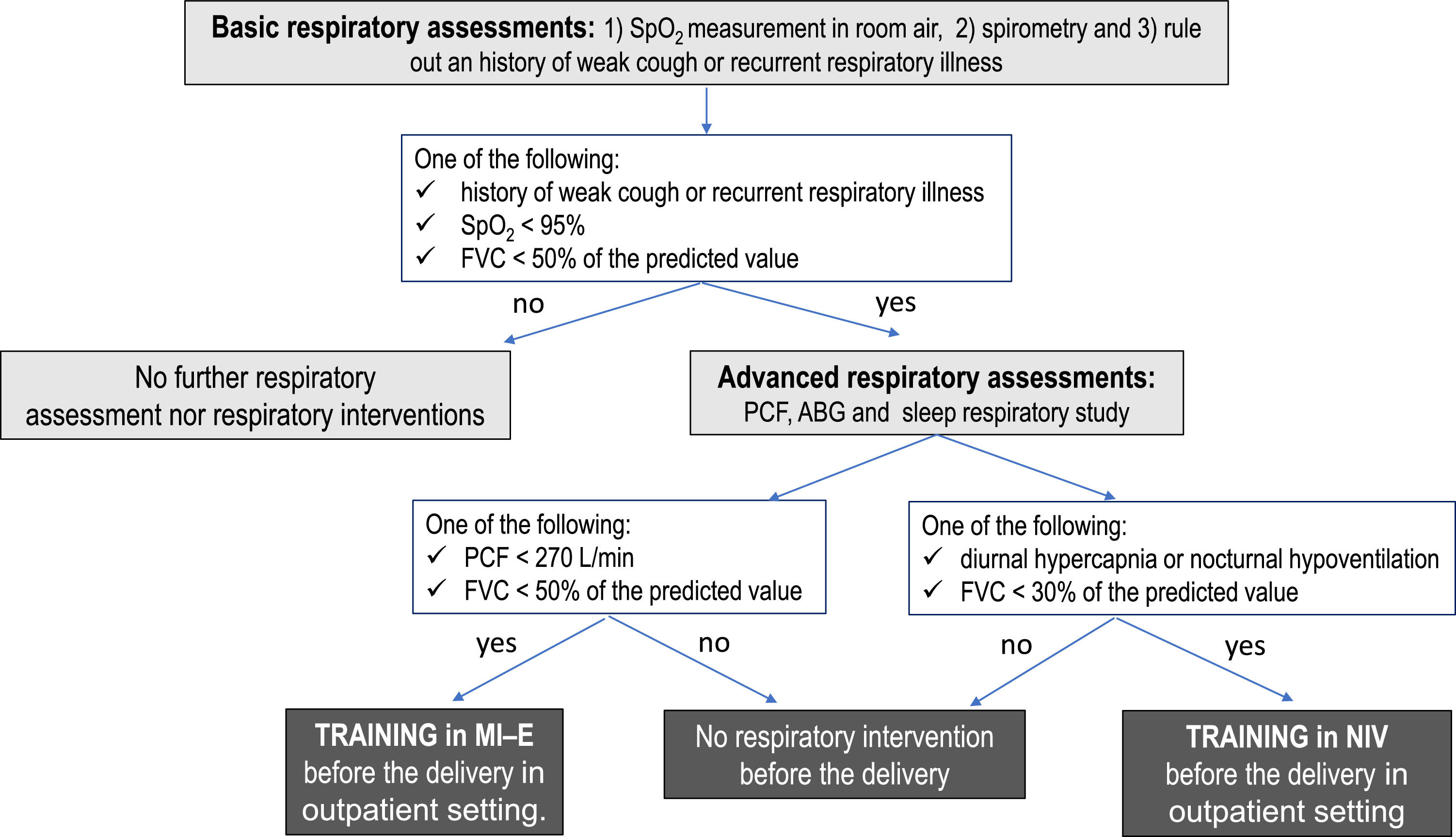
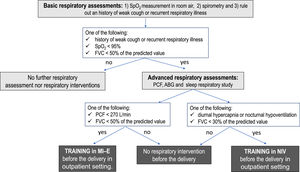
Patients were approached at the 28th-30th week of pregnancy. A multidisciplinary approach was essential in the management of these patients. Our protocol included respiratory tests to assess gas exchange, lung volumes and cough effectiveness as well as training to NIV and MI-E (Figure 1). All women also underwent a neurological assessment to confirm the diagnosis, and, when feasible, the genetic diagnosis was recorded. In addition, all women with myopathies underwent careful assessment of heart function including an electrocardiogram and echocardiogram, if not performed in the previous 12 months.
Patients were considered at risk for pulmonary complications if at least one of the following findings was present: i) oxygen saturation at room air (SpO2) <95%; ii) diurnal or nocturnal hypercapnia; iii) central or obstructive apneas; iv) history of weak cough or recurrent respiratory illness; v) long-term NIV; vi) use of cough assistance techniques at home; vii) forced vital capacity (FVC) < 50% of predicted value; viii) peak cough flow (PCF) < 270 L/min; ix) Gilardeau dysphagia score > 1; x) Cobb angle ≥ 50° Pre-existing respiratory device dependency was also taken into account. Swallowing was evaluated by Gilardeau dysphagia score.25
PCs included any of the following conditions occurring within 7 days after delivery: i) pneumonia, ii) bronchospasm, iii) acute respiratory failure, iv) secretion retention, v) atelectasis, vi) pneumothorax, vii) pleural effusion. They also encompassed: i) invasive mechanical ventilation > 48 h after cesarian section, ii) need for re-intubation iii) need for a tracheostomy.
Patients identified at risk for PCs were trained or re-trained to use NIV and/or MI-E before the delivery. In case of intubation for general anesthesia during cesarean section, all patients at risk for PCs were extubated directly to NIV and MI-E. Continuous NIV were then weaned off or back to baseline hours of use per day.
Twenty out of the 81 NMDs pregnant women included in the study were identified as at risk of respiratory complications and were trained or re-trained to use NIV and/or MI-E. Forty-five patients were affected by myopathyies. Our preliminary results (unpublished data) showed that, when NMDs pregnant women with severe respiratory muscle weakness were trained in the use of NIV and/or mucus clearance techniques before pregnancy and were extubated directly to NIV, mother and neonatal outcome was favourable. After delivery, 13 patients required NIV with or without MI-E. The subgroup at risk of respiratory complications had a higher percentage of PCs (5 vs 1), higher ICU admission rate (11 vs 5), longer hospital length of stay (9.5 ± 7.3 days vs 5.1 ± 2.1 days) than those without respiratory risk factors. No woman was tracheostomized or died. This preliminary results also showed that the use of NIV and MI-E was safe in the peri‑partum period. No complications related to MI-E was described in our patients before and after delivery.
With regard to delivery strategies cesarean section was performed in 63 women and 18 patients underwent vaginal delivery. If general anesthesia was required administration of halogenated agents was avoided in women with myopathies to prevent rhabdomyolysis. Fifty-eight out of 63 patients who required cesarean sections were managed with regional anesthesia. General anesthesia was only performed in five cases and in four of them difficult intubation was recorded. In good agreement with the literature,19,26 this data confirmed that tracheal intubation may be challenging in NMDs patients.
The use of regional anesthesia offers a significant advantage in term of avoidance of general anesthesia side-effects and reduction of postoperative respiratory complications.16 However, severe scoliosis may be sometimes present in these women, making it difficult to perform neuraxial blockade.11 In our study only three patients had severe scoliosis and only two patients underwent spinal surgery. As a consequence, neuraxial blockade was performed in most of the patients undergoing cesarian section and epidural analgesia was administered in 12 out of 18 patients who underwent vaginal delivery.
The low number of patients included in our study and the heterogeneity of these disorders with variable disease severity preclude any statistical analysis. As a consequence, the aim of this commentary was to solicit the involvement of other centers in an international collaborative perspective in order to enroll more NMDs women to confirm the efficacy of this protocol and possibly to evaluate cohorts of patients with specific diseases.
In conclusion, our data suggest that MI-E might be safely used in combination with NIV to prevent and treat secretion retention during pregnancy. Including international centers and enroling larger number of patients we could confirm the efficacy of this peri‑partum protocol.