Left-heart dysfunction and pulmonary vasculopathy are increasingly recognized as contributing factors of exercise capacity limitation in interstitial fibrosing lung disease (IFLD). Moreover, the clinical significance of exercise pulmonary hypertension (ePH) in pulmonary and cardiac diseases has been documented, representing a risk factor for decreased exercise capacity and survival, progression to resting pulmonary hypertension (PH) and overall clinical worsening.
We conducted a prospective study aiming at: (a) assessing the prevalence of PH and ePH in a cohort of 40 functionally limited patients with IFLD, (b) determining the post-capillary (postC) or pre-capillary (preC) etiology of either PH or ePH in this cohort, and (c) examining the correlations between invasively and non-invasively measured exercise variables among hemodynamic groups.
Patients and methods40 IFLD patients underwent cardiopulmonary evaluation, including: clinical examination, lung function tests, 6-minute walking test, heart ultrasonography, cardiopulmonary exercise test and, finally, right heart catheterization (RHC). Resting hemodynamic evaluation was followed by the exercise protocol proposed by Herve et al, using a bedside cycle ergometer in the supine position. Abnormal elevation of mean pulmonary artery pressure (mPAP) above 30mmHg during exercise, with respect to abnormal elevation of cardiac output (CO) below 10 L/min (mPAP–CO ratio ⩾3 mmHg·min·L−1) was used to define ePH (Herve et al, 2015). Secondary hemodynamic evaluation involved detection of abnormal pulmonary arterial wedge pressure (PAWP) increase at peak exercise in relation to CO. Specifically, ΔPAWP/ΔCO >2 mmHg/L per minute determined an abnormal PAWP elevation (Bentley et al, 2020).
ResultsAmong the 40-patient cohort, 25% presented postC PH, 37.5% preC PH, 27.5% ePH, with the remaining 10% recording normal hemodynamics. PAWP evaluation during exercise revealed a postC etiology in 4 out of the 11 patients presenting ePH, and a postC etiology in 6 out of the 15 patients presenting resting preC PH. Mean values of non-invasive variables did not display statistically significant differences among hemodynamic groups, except for: diffusing capacity for carbon monoxide (DLCO), carbon monoxide transfer coefficient (KCO) and the ratio of functional vital capacity to DLCO (FVC%/DLCO%), which were lower in both ePH and PH groups (p < 0.05). Resting values of CO, cardiac index (CI), stroke volume (SV) and pulmonary vascular compliance (PVC) were significantly impaired in ePH, preC-PH and postC-PH groups when compared to the normal group.
ConclusionsBoth PH and ePH were highly prevalent within the IFLD patient group, suggesting that RHC should be offered more frequently in functionally limited patients. Diffusion capacity markers must thus guide decision making, in parallel to clinical evaluation. ePH was associated to lower resting CO and PVC, in a similar way to resting PH, indicating the relevance of cardiopulmonary function to exercise limitation. Finally, the use of the ΔPAWP/ΔCO>2 criterion further uncovered PH of postcapillary etiology, highlighting the complexity of hemodynamics in IFLD.
ClinicalTrials.gov ID: NCT03706820
Left-heart dysfunction and pulmonary vasculopathy are increasingly recognized as factors of exercise capacity limitation in interstitial fibrosing lung disease (IFLD) along with respiratory limitation.1,2
Pulmonary hypertension (PH) can occur as a complication of IFLD, negatively affecting patients’ functional capacity and prognosis.3,4 A thorough cardiopulmonary evaluation including right heart catheterization (RHC) is therefore needed, to properly diagnose and differentiate between pre- and post-capillary (preC and postC, respectively) PH. Moreover, the implementation of an exercise protocol during RHC is gaining ground in the field of IFLD and of other pulmonary and cardiac diseases, potentially promising the prompt detection of pulmonary vasculopathy.2,5,6
The criterion of abnormal elevation of mean pulmonary artery pressure (mPAP) above 30 mmHg during exercise, with respect to abnormal elevation of cardiac output (mPAP–CO ratio ⩾3 mmHg·min·L−1) is increasingly being used for the definition of exercise pulmonary hypertension (ePH).7,8 Furthermore, criteria to define abnormal pulmonary artery wedge pressure (PAWP) elevation during exercise are currently being established, contributing to an integrated understanding of exercise vascular responses.9 The clinical significance of ePH in pulmonary and cardiac diseases has been documented, representing a risk factor for decreased exercise capacity and survival, progression to PH and overall clinical worsening.10–14
Driven by these trends, we conducted a prospective study aiming at: (a) assessing the prevalence of PH and ePH in a cohort of 40 functionally limited patients with IFLD, (b) determining the postC or preC etiology of either PH or ePH in this cohort, and (c) examining the correlations between invasively and non-invasively measured exercise variables among hemodynamic groups. Among the few existing studies assessing the pulmonary vascular responses to exercise in IFLD patients, our study is the first to also investigate the prevalence of postC etiology of PH and ePH, using resting and exercise RHC, thus providing distinct phenotypes of exercise limitation in IFLD.
MethodsStudy design and populationThis prospective study was approved by the ethics board of the Aristotle University of Thessaloniki, Greece, and informed consent was obtained by all subjects. A consecutive population of 40 Caucasian patients presenting with pulmonary fibrosis, either idiopathic or secondary to connective tissue disease (CTD)15-17 and met the inclusion/exclusion criteria, were evaluated between May 2018 and March 2020 at the Pulmonary Hypertension Outpatient Clinic of the Department of Respiratory Failure, in a tertiary university hospital. Diagnosis of the underlying etiology of the fibrotic lung disease was established at the Interstitial Lung Disease Outpatient Clinic of the University Respiratory Department, according to international guidelines.15-17 Patients underwent cardiopulmonary evaluation, including clinical examination, lung function tests, 6-minute walking test (6MWT), heart ultrasonography, and cardiopulmonary exercise test (CPET), unless contraindicated, during their initial visit. A second visit was scheduled one to two weeks after the first, to perform RHC. Resting hemodynamic evaluation was followed by the exercise protocol proposed by Herve et al, using a bedside cycle ergometer in the supine position.8 Patients with resting postC PH were excluded from the exercise protocol.
Exclusion criteria were as follows: 1) functional vital capacity (FVC) <50% of predicted values, 2) presence of radiologic evidence of emphysema and/or spirometric evidence of airway obstruction, 3) contraindications for exercise tests, such as unstable angina, symptomatic arrythmia, severe hypoxemia, musculoskeletal disease etc., 4) recent acute myocardial infarction or pulmonary embolism in the past year, 5) presence of moderate or severe valvular disease or left ventricular ejection fraction < 50%, 6) treatment with specific PH agents.
Hemodynamic measurementsContinuous monitoring of vital signs was initiated prior to RHC, including electrocardiograms, pulse oximetry and blood pressure measurements by a cuff sphygmomanometer at frequent intervals. Supine RHC was performed by percutaneously inserting a balloon-tipped, triple-lumen, fluid-filled Swan-Ganz pulmonary arterial catheter via the internal jugular vein. Complete hemodynamic and oxygen profiles were obtained at rest. Zero level was set at midthoracic level.18 Resting pressures were measured at the end of expiration.19 CO was measured by thermodilution and expressed as the mean of three measurements at rest, and of two during exercise.
During exercise RHC, patients were instructed to cycle at a rate of 60 revolutions per minute until first occurrence of any of the following: exhaustion, discomforting dyspnea or chest ache (flagged by the patient), or an observed arterial oxygen saturation of <80%. Measurements of systolic, diastolic and mean pulmonary artery pressure (sPAP, dPAP, mPAP, respectively), PAWP and CO were obtained at unloaded pedaling (0 W) and at constant workload increments of 10 W. Each stage lasted 3 minutes and values were obtained on the second minute of each stage. Pressure waveforms were averaged over three respiratory cycles, and vital signs were also recorded for each stage. Total pulmonary resistance (TPR) was defined as the mPAP to CO ratio at maximal exercise and expressed as Wood units (WU).8,20 Pulmonary vascular compliance (PVC) was calculated by the ratio of stroke volume (SV) to (sPAP-dPAP). The ratio of PAWP to CO (ΔPAWP/ΔCO) was calculated as follows: PAWP(peak)−PAWP(rest)CO(peak)−CO(rest)9 .
Hemodynamic definitionsResting PH was defined by mPAP ≥25 mmHg; please note that the revised PH definition of mPAP ≥20 mmHg arose after the initiation of the study.21 PAWP ≤15 mmHg defined preC-PH at rest, whereas postC-PH was defined as PAWP>15 mm Hg.3 ePH was defined as mPAP >30 mmHg and TPR > 3 mmHg·min·L− 1 at maximal exercise.8 ΔPAWP/ΔCO ≤2 mmHg/L per minute determined a preC etiology, while ΔPAWP/ΔCO >2 mmHg/L per minute a postC etiology.9
Statistical analysisStatistical analysis was performed using SPSS software, version 23 (IBM SPSS Statistics, IBM Corporation). Values are reported as mean ± standard deviation (SD) or median [interquartile range]. Normality of distribution was assessed by the Shapiro-Wilk test. Normally distributed continuous variables were compared between groups by using a one-way analysis of variance (ANOVA) with a Scheffe post hoc analysis. Nonnormally distributed continuous variables were compared among groups by using the Kruskal-Wallis test with Dunn's post hoc test. Categorical variables were compared between groups by using a chi-squared test. Alterations of hemodynamic variables from rest to exercise were evaluated using paired t-tests. Correlations between variables were assessed using Pearson's r or Spearman's rho correlation coefficients, depending on the data distribution. P values of < 0.05 were considered statistically significant.
ResultsDemographic and functional characteristicsForty (N = 40) IFLD patients were evaluated. Mean age was 68±11 years, and pulmonary restriction was variable, from mild to moderate. Patients were equally distributed across NYHA classes II, III and IV. Idiopathic pulmonary fibrosis (IPF) was diagnosed in 75% of the patients (n = 30), with lung fibrosis secondary to CTDs among the remaining 25% (systemic sclerosis, n = 6, rheumatoid arthritis, n = 3, systemic lupus erythematosus, n = 1). All CTD patients and 11 of the IPF patients were not receiving an antifibrotic agent, while use of nintedanib was reported by 10 of the IPF patients and use of pirfenidone by 7. Radiologic severity of fibrosis22,23 was mostly moderate (50%, n = 20), with mild fibrosis documented for 35% and severe for 15%. Patients’ characteristics grouped by initial hemodynamic allocation are summarized in Table 1.
Patient characteristics grouped by initial hemodynamic allocation.
Data are expressed as mean±SD, or median [interquartile range] and only data in bold are statistically significant (p < 0.05). 6MWT=6-minute walking test; ΔSPO2 = arterial oxygen desaturation; Ao= aorta diameter; BMI = body mass index; CTD = connective tissue disease; DLCO=diffusing capacity for carbon monoxide; FVC= forced vital capacity; FEV1 = forced expiratory volume in one second; HRCT = high resolution computed tomography; IPF = idiopathic pulmonary fibrosis; KCO = carbon monoxide transfer coefficient; LTOT = long term oxygen treatment; LVEDD = left ventricular end-diastolic diameter; LVEF = left ventricular ejection fraction; PA= pulmonary artery diameter; RV = residual volume; RVEDD = right ventricular end-diastolic diameter; RA = right atrial area; RVSP = right ventricular systolic pressure; TLC = total lung capacity; TRV = tricuspid regurgitant jet velocity.
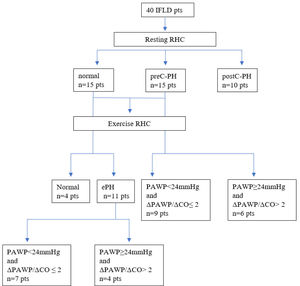
Initial hemodynamic evaluation allocated patients into four groups: (a) normal group (n = 4, 10%), presenting normal hemodynamics at rest and exercise, (b) ePH group (n = 11, 27.5%), presenting normal resting hemodynamics and ePH, (c) preC-PH group (n = 15, 37.5%), presenting preC-PH at rest, and (d) postC-PH group (n = 10, 25%); presenting post-C PH at rest. The postC-PH group did not undergo further exercise hemodynamic evaluation.
Secondary hemodynamic evaluation involved detection of abnormal PAWP increase at peak exercise in relation to CO (ΔPAWP/ΔCO> 2mm Hg/L per minute), as stated in Methods, thus dividing the ePH and preC-PH groups into the following subgroups: (i) preC-ePH group (n = 7), presenting preC etiology of ePH, (ii) postC-ePH group (n = 4), presenting postC etiology of ePH, (iii) preC-PH-PAWP (n = 9), presenting preC etiology of PH, (iv) preC-PH+PAWP (n = 6), presenting postC etiology of PH. A cut-off value of 24mmHg for peak PAWP differentiating preC and postC etiology was driven by the implementation of the main stated criterion (ΔPAWP/ΔCO> 2mm Hg/L per minute). The hemodynamic allocation of the study cohort is illustrated with a tree diagram in Fig. 1.
Comparisons of non-invasive variables among groupsMean values of non-invasive variables did not display statistically significant differences between hemodynamic groups, except for diffusion capacity markers (DLCO, KCO and FVC%/DLCO%), which were lower in both ePH and PH groups (p < 0.05). FVC%/ DLCO% ratio has been evaluated as a noninvasive predictor for the development of PH in patients with interstitial lung disease and the cutoff value of 1.39 has been measured to offer 96% sensitivity and 65% specificity for this predictive purpose.24 Non-invasive variables grouped by initial hemodynamic allocation are summarized in Table 1.
All patients in the postC-PH group had at least one documented cardiac comorbidity (arterial hypertension, coronary artery disease) and echocardiographic evidence of diastolic dysfunction with preserved ejection fraction.25 Cardiac comorbidities were more common in the postC-ePH and preC-PH+PAWP subgroups, compared to preC-ePH and preC-PH-PAWP subgroups respectively [69% vs 42%, (p = 0.03) and 72% vs 47%, (p = 0.04)]. Comparisons of spirometric values among the four subgroups indicated milder pulmonary restriction in the subgroups with abnormal PAWP elevations than those without. Specifically, mean FVC% was 79.4±17.28% in the postC-ePH subgroup versus 61.5±12.46% in the preC-ePH subgroup (p < 0.05), and 83.85±21.13% in the preC-PH+PAWP subgroup versus 64.31±12.28 in the preC-PH-PAWP subgroup (p < 0.05).
CPETStatistical analysis of variables obtained from CPET did not produce statistical differences between the various groups, possibly due to small study sample, as only 22 patients performed CPET. Main reason of CPET exclusion was resting hypoxia of patients receiving long-term oxygen treatment (LTOT). Peak oxygen consumption (VO2) at anaerobic threshold (AT) and at peak exercise was reduced in the majority of patients. Ventilatory reserve was abnormal (<11lt) in 7 patients (2 preC, 2 postC, 2 ePH, and 1 normal). The ratio of minute ventilation to carbon dioxide output at anaerobic threshold (VE/VCO2 at AT), which has been validated as a detector of pulmonary vasculopathy,26 was normal in all patients of the normal group and abnormal (>34) in all patients of the ePH and PH groups.
Comparisons of resting and exercise hemodynamic variables among groupsResting values of CO, cardiac index (CI), stroke volume (SV) and pulmonary vascular compliance (PVC) were significantly impaired in the ePH, preC-PH and postC-PHs groups when compared to the normal group. However, these variables were similar between the ePH, preC-PH and postC-PH groups. Mean and p values of hemodynamic variables are summarized in Table 2 (for resting supine RHC) and Table 3 (for exercise supine RHC).
Resting supine right heart catheterization.
Data are expressed as mean±SD. CI = cardiac index; CO = cardiac output; dPAP = diastolic pulmonary artery pressure, mPAP = mean pulmonary arterial pressure; PAWP = pulmonary arterial wedge pressure; PVC = pulmonary vascular compliance; PVR = pulmonary vascular resistance; RAP = right atrial pressure; sPAP = systolic pulmonary artery pressure; SV = stroke volume; SvO2= oxygen saturation of central venous blood; SVR = systemic vascular resistance; TPG = transpulmonary gradient; TPR = total pulmonary resistance.
Exercise supine right heart catheterization.
Data are expressed as mean±SD. CI= cardiac index; CO= cardiac output; dPAP = diastolic pulmonary artery pressure, mPAP = mean pulmonary arterial pressure; PAWP = pulmonary arterial wedge pressure; PVC = pulmonary vascular compliance; PVR = pulmonary vascular resistance; RAP = right atrial pressure; sPAP=systolic pulmonary artery pressure; SV = stroke volume; SaO2 = oxygen saturation of arterial blood; SVR = systemic vascular resistance; TPG = transpulmonary gradient; TPR = total pulmonary resistance.
The current study performed pulmonary hemodynamic measurements at rest and at exercise in a stable population of functionally limited IFLD patients with various degrees of respiratory restriction, identifying a high prevalence of ePH (27.5%) and PH (preC=37.5%, postC=25%). The ePH phenotype displayed lower resting CO and CI values, indicating the contribution of cardiopulmonary function in exercise limitation. In line with our findings, a recent study with invasive CPET performed to 27 ILD patients also supports that ePH is associated with lower peak VO2 and CO, increased dead space ventilation and inefficient ventilation during exercise.5
Several pathophysiologic mechanisms, such as vasculopathy of the small pulmonary arteries, interstitial lung disease or myocardial fibrosis leading to left ventricular dysfunction, are known to produce PH in CTD patients, especially in systemic sclerosis.27 The aforementioned mechanisms also exist in IPF patients.28-31 Vascular remodeling can occur due to overexpression of cytokines and growth factors, while several cardiac manifestations are present, including arrythmias and coronary artery disease, making diastolic dysfunction common in those patients.27,28
Current guidelines do not endorse the use of vasoactive agents for PAH neither in lung disease associated PH, nor in ePH, as there is lack of strong evidence. However, ongoing research increasingly focuses towards carefully selecting patients that could benefit from such treatments, while excluding others that might instead deteriorate, such as patients with left heart disease. With that in mind, we believe that phenotyping exercise hemodynamics could contribute to appropriate patient clustering in future research. We also believe that the identification of left ventricular diastolic dysfunction as a factor of functional limitation is highly significant, as it can improve with medical treatment and lifestyle changes. We frequently observe in our clinical practice that the presence of severe lung disease draws the attention away from routine cardiovascular care.
This was the motivation behind examining PAWP responses to exercise in this study. We interpreted exercise-induced increases in PAWP with respect to flow, using the ratio of ΔPAWP/ΔCO>2, as proposed by recent well-designed reports.9,21 Exercise measurements increased postC diagnoses by 25%, as 6 patients of the preC-PH group and 4 patients of the ePH group presented an abnormal elevation of PAWP during exercise. The above reclassified patients were more likely to present at least one relevant comorbidity (p = 0.03; arterial hypertension, atrial fibrillation, coronary artery disease, diabetes, obesity) and higher FVC% when compared to patients without an abnormal PAWP response of the same initial hemodynamic group (80% vs 65%, p < 0.05). These findings could describe a clinical phenotype with occult left heart disease. Therefore, exercise RHC should be offered to functionally limited IFLD patients with cardiac comorbidities and mild restriction to evaluate the contribution of heart and lung disease to exercise limitation.
The overall prevalence of postC diagnoses in our cohort reached 50% of the total patients and contrasts with previous studies in IPF reporting prevalence of 9-16%,32,33 without including exercise RHC. However, an abnormal PAWP exercise response might not necessarily be the result of left ventricular diastolic dysfunction. It might also be attributed to right ventricular pressure overload and subsequent interventricular septal pressure load on left ventricular function.34 Therefore, the above finding should be interpreted with caution. The complete hemodynamic profile of each patient has to be always considered, along with clinical and echocardiographic features. In addition, we must state that the exercise protocol in the resting PH group has a clinical role in examining PAWP responses, but is still under investigation, requiring high levels of expertise to be executed and interpreted for lung disease patients.
Our study also produced agreeable results with previous studies concerning PVC that found it to be reduced in both ePH and PH phenotypes. PVC is an early marker of pulmonary vasculopathy, as derangement occurs before increases in PVR are evident during the natural history of PH.35,36 Similarly, a recent study evaluating the changes in PVC relative to PVR during exercise in ILD patients confirmed that PVC deteriorates prior to PH diagnosis.2
Furthermore, the poor correlation in IPF between PH and lung function markers has been largely known4,5,37,38 and aligns with our results, where no such association was observed. The sole exception were diffusion capacity markers that presented a strong association to pulmonary vasculopathy; a finding also consistent with existing literature.39,40
Overall, non-invasive markers of pulmonary vascular disease produced by echocardiography and CPET can guide clinical practice, but fail to accurately predict ePH and PH, as already evident in existing literature.41-44 Echocardiographic features, such as right atrial enlargement and estimated right ventricular systolic pressure have been found to correlate to pulmonary vasculopathy in PH associated to lung disease.41,43 Similarly, evaluation by CPET can discriminate among pulmonary vasculopathy, respiratory limitation, and left heart disease.44,45
Strengths and limitationsAmong the strengths of this study are the prospective nature of data collection, the use of the gold standard RHC and the fact that the same team of physicians conducted all evaluations, minimizing technique and interpretation variability.
Conversely, a study limitation is the small monocentric study sample, largely due to the rarity of the disease, which impacts the statistical power of the analysis. The normal group is much smaller from the other groups, though we included patients shortly after diagnosis. A selection bias might exist as patients with limited symptoms tend to be less willing to undergo further evaluation that includes an invasive examination.
Age-related limits to hemodynamic normality for the diagnosis of ePH might also be reasonably questioned. Although TPR appears to have high specificity and sensitivity for age groups both below and above 50 years of age, diagnosis of ePH is problematic for those aged above 70 years, as abnormal exercise hemodynamics could be the result of normal ageing.8,46 Given our study population has a mean age approximating this cut-off value, our results should be interpreted with caution.
Finally, precision in measurements of pressures during RHC is globally challenging, as movement artifacts and intrathoracic pressure swings can affect hemodynamic waveforms, especially during exercise.19 That was the reason behind excluding patients with radiologic evidence of emphysema and/or airflow limitation of any degree, to avoid overestimation of pressures due to significant air trapping.47,48
ConclusionThe present study analyzed rest and exercise pulmonary hemodynamics of a real-life cohort of IFLD patients with varying degrees of pulmonary restriction. Both rest and exercise PH were highly prevalent, suggesting that RHC should be offered more frequently in functionally limited patients. Diffusion capacity markers must guide decision making, in parallel to clinical evaluation. ePH was associated to lower resting CO and PVC, in a similar way to resting PH, indicating the relevance of cardiopulmonary function to exercise limitation. Finally, the use of the ΔPAWP/ΔCO>2 criterion further uncovered postcapillary etiology of PH and ePH, highlighting the complexity of hemodynamics in IFLD. However, further studies with a more homogeneous patient population are needed to confirm these findings.
I declare that I accept to undertake all the responsibility for authorship during the submission and review stages of the manuscript.