Bronchoalveolar lavage (BAL) with quantitative cultures has been used in order to increase ventilator associated pneumonia (VAP) diagnosis specificity, although the accurate technique for this entity diagnosis remains controversial.
ObjectivesTo evaluate the influence of using positive BAL and quantitative cultures results in microbiologic diagnosis and treatment of patients with suspected late VAP and prior antibiotherapy.
Material and methodsRetrospective analysis of intensive care unit (UCI) patients, during a one year period, with clinical suspicion of late VAP and prior use of antibiotics that presented a growth in BAL cultures.
ResultsOf 243 BAL performed, there were 71 (29.2 %) positive cultures (60 patients, 76.7 % male, 54±19 years). BAL was done after 13 days (median) of invasive mechanical ventilation, 11 days of ICU antibiotherapy and in the day in which a new antibiotic for VAP suspicion was started. Colony forming units (CFU)/ml count was performed in 71.8 % and endotracheal aspirate (ETA) simultaneously collected for qualitative analysis in 85.9 %. Therapeutic approach was changed in 38.0 %: correction (16.9 %), de-escalation (12.7 %) and directed antibiotherapy start (8.4 %). Therapeutic changes were made in the presence of CFU>104 in 84.2 % and in agreement with ETA in 70.8 %. In cases in which antibiotherapy was maintained (62.0 %), quantitative cultures would have allowed de-escalation in 9.1 %. Changes in prescription were more frequent when CFU was>104 (48.5 %), comparing with situations in which counts were lower and BAL analysis was only qualitative (28.9 %), p=0.091. There were no significant differences between patients submitted to different therapeutic approaches concerning to ICU mortality or length of stay.
ConclusionIn late onset VAP, positive BAL and quantitative cultures allowed therapeutic changes, leading to antibiotic adequacy and consumption reduction, which can however be maximised.
O lavado broncoalveolar (LBA) com culturas quantitativas tem sido utilizado no sentido de permitir um aumento da especificidade diagnóstica da pneumonia associada ao ventilador (PAV), embora a técnica padrão para o diagnóstico definitivo desta entidade permaneça controversa.
ObjectivosAvaliar a influência dos resultados positivos do LBA e das culturas quantitativas no diagnóstico microbiológico e no tratamento de doentes com suspeita de PAV tardia e antibioterapia prévia.
Material e MétodosAnálise retrospectiva de doentes em unidade de cuidados intensivos (UCI) polivalentes, com suspeita de PAV tardia e antibioterapia prévia, que apresentaram crescimento cultural nos LBA efectuados durante o período de um ano.
ResultadosDos 243 LBA realizados, obtiveram-se 71 (29,2 %) resultados positivos (60 doentes, 76,7 % do sexo masculino, 54±19 anos). O LBA foi realizado após em mediana 13 dias de ventilação invasiva, 11 dias de antibioterapia na UCI e no dia em que se instituiu um novo antibiótico perante a suspeita de PAV. Foi efectuada contagem de unidades formadoras de colónias (UFC)/ml em 71,8 % e simultaneamente obtido aspirado endotraqueal (AET) para análise qualitativa em 85,9 %. Verificou-se mudança terapêutica em 38,0 %: correcção terapêutica (16,9 %), descalação (12,7 %) e início de antibioterapia dirigida (8,4 %). As alterações terapêuticas foram efectuadas na presença de>104 UFC em 84,2 % e em concordância com o AET em 70,8 %. Nos casos em que se verificou manutenção da antibioterapia (62,0 %) as culturas quantitativas teriam permitido descalação em 9,1 %. A alteração na prescrição foi mais frequente na presença de>104 UFC (48,5 %), do que nas situações em que as contagens de UFC foram inferiores ou a análise do LBA qualitativa (28,9 %), p=0,091. Não se verificaram diferenças significativas entre os doentes submetidos às diferentes atitudes terapêuticas no que diz respeito à mortalidade na UCI ou duração do internamento.
ConclusãoNa PAV tardia, o resultado positivo do LBA e das culturas quantitativas condicionou mudanças terapêuticas, no sentido da adequação da antibioterapia e da redução do consumo de antibióticos, que podem contudo ser maximizadas.
Ventilator associated pneumonia (VAP) is the most common nosocomial infection acquired in intensive care unit (ICU), occurring in 8 to 28 %1 of patients under invasive mechanical ventilation (IMV) for 48 hours or more. It is also an independent mortality factor, contributing to an estimate attributable mortality rate of 20 to 30 %.2
VAP diagnosis remains a challenge for the clinician, being essential for early and adequate antibiotherapy institution, assumptions that are significantly associated with hospital mortality reduction.3,4
Clinical diagnosis approach based on conjugation of clinical, radiologic and laboratory criteria5 demonstrated sensitivity and specificity values (69 % and 75 %, respectively) lower than desirable,6 often leading to an over estimation of disease incidence, due to difficulties in differentiating colonization from infection and in differentiating non infectious diseases that can mimic VAP findings.
Besides diagnosis difficulty, VAP antibiotherapy selection is conditioned by the possibility of potentially multidrug resistant organisms (MDRO) infection, agents particularly involved in late-onset pneumonia.7,8 Therefore, it is essential to obtain an assertive microbiologic diagnosis allowing an increase of VAP specificity diagnosis and adequacy of initial therapy and, whenever possible, to reduce antibiotics consumption, their side effects and pressure selection.
In this context, different microbiologic approaches have been used, namely invasive like bronchoalveolar lavage (BAL) and quantitative cultures. Although literature data about their value are controversial and despite two decades of research, there is still not a gold standard technique for VAP definitive diagnosis.
Quantitative cultures have been used to help overcome difficulties in distinguishing colonization from infection. Threshold of 104 colony forming unit (CFU)/ml in BAL presents sensitivity and specificity values that range between 42–93 % and 45–100 % respectively, higher than those described for endotracheal aspirate (ETA) 106 threshold, between 36–82 % and 72–85 %.7
Among studies that compare the impact of invasive techniques with quantitative cultures to those non-invasive,9–18 only five are controlled and randomized.12–16 A French group showed statistically significant differences with more antibiotic-free days and reduced mortality at 14th day in patients included in the invasive strategy. 15 Afterwards, a meta-analysis concluded that the use of invasive techniques affected antibiotic prescription but not mortality. 19 More recently, a Canadian study found no differences in antibiotic use and outcomes in patients submitted to any of these technical approaches.16
Variability in studies design, their frequent power reduced sample size and heterogeneity of criteria applied to populations, may partly explain the diversity of literature data.17,19
Despite controversy, scientific evidence demonstrating benefit in invasive techniques use seems to exist and they are recommended in some consensus documents,7,8 namely if procedure is feasible and biological sample is viable.8
The authors performed a preliminary study with the main goal of evaluating the influence of BAL positive results and quantitative cultures in microbiologic diagnosis and treatment of suspected late VAP in patients with prior antibiotherapy. As a secondary objective, the authors analyzed ICU mortality and length of stay.
Material and methodsA retrospective analysis of clinical files of patients admitted in two adults multidisciplinary Intensive Care Units (28 beds) of a university hospital, which had positive results on BAL performed in a one year period (September 2007–2008) was carried out.
All patients had clinical suspicion of VAP, were admitted for more than four days and were previously submitted to antibiotherapy (minimum duration of two days, prophylaxis included).
VAP was suspected in the presence of a new or worsened radiological infiltrate associated with at least two of the following three criteria: purulent respiratory secretions, temperature of over 38.° C or under 36.° C, leukocyte count of over 10,000/mm3 or leukopenia under 4,000/mm3.
BAL was obtained by flexible bronchoscopy (FBC) as the following protocol: 1) adequate patient sedation and/or curarization; 2) fraction of inspired oxygen of 100 %; 3) introduction of bronchoscope through endotracheal tube using a special adaptor; 4) avoidance of suctioning and local anesthetic use; 5) wedge of the bronchoscope into a subsegmental bronchus corresponding to the area of radiologic abnormality; 6) sequential instillation of six aliquots of sterile saline (20ml each) and slow manual aspiration; 7) rejection of the first aliquot; 8) microbiologic assessment of BAL fluid obtained (minimum 40–60ml) in one hour maximum period. BAL was analysed in a quantitative or qualitative way.
ETA was collected by aseptic technique, within 24 hours of BAL achievement, and processed, in all cases, qualitatively. Growth of one or more microorganisms in quantitative or qualitative cultural exams of these biological products was considered as a positive result.
Therapeutic considerations were based on an ICU antibiotic therapy protocol. Concepts of maintenance and change of therapeutic approach were used, the latter including adjustment/correction of inappropriate treatment, de-escalation and beginning of directed antibiotherapy. Inappropriation was defined by identification of a non suspected agent at time of empiric therapy institution or of a microorganism whose antimicrobial susceptibility test showed resistance to at least one, two in case of P. aeruginosa, prescribed antibiotics. De-escalation could have included reduction in the number of antibiotics, narrowing of antibiotic spectrum, reduction of duration or discontinuation of antibiotherapy.
Results were presented as percentage for categorical and median (interquartile range) or mean (± standard deviation) for continuous variables. Statistical analysis was performed using SPSS, version 16.0. Chi square and Fisher tests were used for proportions, and Mann–Whitney U and Kruskal-Wallis H tests for continuous variables. Statistical significance was set to a value of p ≤ .05.
ResultsGeneral characteristics of populationWe analyzed 71 BAL positive cultural results, performed in 60 patients, 76.7 % (n = 46) males, with an average age of 54 ± 19 years (Table 1).
General characteristics of study population (n = 60)
| Age, years | 54 ± 19 |
| Male sex, n (%) | 46 (76.7 %) |
| SAPS II | 48 (34) |
| Causes of admission, n (%) | |
| Medical | 35 (58.3 %) |
| Trauma | 15 (25.0 %) |
| Surgical | 10 (16.7 %) |
| Diagnosis group, n (%) | |
| Respiratory | 18 (30.0 %) |
| Trauma | 15 (25.0 %) |
| Neurologic | 9 (15.0 %) |
| Cardiovascular | 7 (11.7 %) |
| Gastrointestinal | 7 (11.7 %) |
| Renal | 3 (5.0 %) |
| Others | 1 (1.7 %) |
| On date of FBC | |
| Temperature (.°C) | 37 (2) |
| Leukocytes (cels/mm3) | 13243 (7) |
| Pa02/Fi02 | 220 (87) |
| Shock, n (%) | 14 (23.3 %) |
| Multiple infections, n (%) | 37 (61.7 %) |
| Prior antibiotherapy, n (%) | 60 (100.0 %) |
| Prior 24h antibiotherapy, n (%) | 54 (90.0 %) |
| Duration of IMV, days | 13 (12) |
| Duration of previous antibiotherapy, days | 11 (11) |
FBC: flexible bronchoscopy; Fi02: inspired oxygen fraction; IMV: invasive mechanical ventilation; SAPS II: simplified acute physiology score II.
Nine patients underwent a second BAL, and in two of these, there was a third BAL (median time period, 12 [15] days).
BAL was done after 13 (12) days of IMV, after 11 (11) days of ICU antibiotherapy and in day 1 (5) of new antibiotherapy for VAP suspicion.
Another simultaneously infectious foci was detected in 61.7 % (n = 37) of the patients. Immunosuppression was present in 13.3 % (n = 8).
All patients were submitted to prior antibiotic therapy and only 10.0 % (n = 6) were not under antibiotics within 24 hours before BAL attainment.
Microbiologic resultsIn one year, 243 BAL were performed, with a microbiological yield of 29.2 % (n = 71).
A single pathogen was found in 85.9 % (n = 61) and polymicrobial in the remaining 14.1 % (n = 10). Pseudomonas aeruginosa (33.8 %, n = 24), Enterobacteriaceae (19.7 %, n = 14) and methicillin-resistant Staphylococcus aureus (MRSA) (16.9 %, n = 12) were the most frequently isolated agents, followed by fungi, (15.5 %, n = 11), namely Candida spp (n = 10) and Aspergillus spp (n = 1). Others included,
Acinetobacter baumannii (4.2 %, n = 3), methicilin susceptible Staphylococcus aureus (4.2 %, n = 3), Enterococcus faecium (2.8 %, n = 2), Haemophilus influenzae (1.4 %, n = 1) and Stenotrophomonas malthophilia (1.4 %, n = 1).
ETA was simultaneously collected in 85.9 % (n = 61). In 71.8 % (n = 51) BAL CFU/ml count was performed: > 104 in 46.5 % (n = 33), between 104-103 in 19.7 % (n = 14) and < 103 in 5.6 % (n = 4). BAL analysis was only qualitative in the remaining 28.2 % (n = 20) episodes.
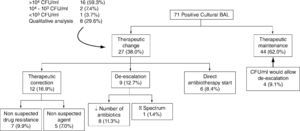
Therapeutic approachAfter knowing cultural BAL result, therapeutic approach was maintained in 62.0 % (n = 44) and changed in 38.0 % (n = 27), namely with adjustment/correction of therapy in 16.9 % (n = 12), de-escalation in 12.7 % (n = 9) and start of directed antibiotherapy in 8.4 % (n = 6) (Fig. 1).
In 59.3 % (n = 16/27) of cases in which therapeutic approach was changed CFU/ml count was > 104, in 7.4 % (n = 2/27) between 104-103, in 3.7 % (n = 1/27) < 103 and in 29.6 % (n = 8/27) was based on BAL qualitative analysis. There was agreement between BAL and ETA results in 63.0 % (n = 17/27), disagreement in 25.9 % (n = 7/27) and in 11.1 % (n = 3/27) we did not collect the last biologic sample. Analyzing these results to all quantitative cultures and ETA obtained, we found that therapeutic approach was changed in the presence of CFU > 104 in 84.2 % (n = 16/19) and in agreement with ETA in 70.8 % (n = 17/24).
In all cases of performance of more than one BAL, therapeutic attitude was maintained, except in one case, in which de-escalation was made.
Inappropriate empirical antibiotherapy adjustment/correction resulted in 58.3 % (n = 7/12) of a non suspected drug resistance (P. aeruginosa [n = 4], A. baumannii [n = 1], Enterobacteriaceae [n = 1] and Enterococcus spp. [n = 1]) and in 41.7 % (n = 5/12) of a non suspected agent isolation (fungi [n = 3], MRSA [n = 1] and Enterobacteriaceae [n = 1]).
Klebsiella and Enterobacter spp. isolated in two patients on therapy with an Aminopenicillin were the above mentioned Enterobacteriaceae. Candida and Aspergillus spp. occurring in patients with severe sepsis and immunosuppression related factors (systemic erythematosus lupus, asplenia and HIV infection) were the non suspected isolated fungi.
De-escalation included both reduction in the number of antibiotics in 88.9 % (n = 8/9) (vancomycin [n = 3], carbapenem [n = 2], quinolone and aminoglycoside [n = 1], vancomycin and aminoglycoside [n = 1] and colistin [n = 1]) and narrowing of antibiotic spectrum (ureidopenicillins to 3rd generation cephalosporin) in 11.1 % (n = 1/9).
In 8.4 % (n = 6) of cases, antibiotherapy was started only after knowing BAL result: P. aeruginosa (n = 5) and Enterobacteriaceae (n = 1).
In cases in which antibiotherapy was maintained, quantitative cultures would have allowed de-escalation in only 9.1% (n = 4/44).
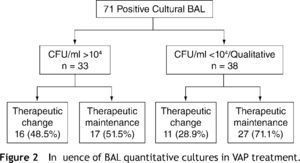
Analyzing the influence of quantitative BAL cultures in the therapeutic approach, we found that change in prescription was more frequent when CFU was > 104 (48.5 %, n = 16/33) than in situations in which counts were lower and BAL analysis was qualitative (28.9 %, n = 11/38), p = .091 (Fig. 2).
OutcomesCrude mortality in the 60 patients was 21.7 % (n = 13). The cause of death was directly attributed to pneumonia in 11.7 % (n = 7), 14.7 % (n = 5/34) in the group in which therapeutic approach was maintained and 7.7 % (n = 2/26) in the group in which it was changed, p = .688 (Table 2).
ICU mortality according to different therapeutic approaches (n = 60)
| Antibiotic therapy | Change (n = 26) | Maintenance (n = 34) | p |
| Crude mortality, n (%) | 8 (30.8 %) | 5 (14.7 %) | 0.206 |
| VAP atributable mortality, n (%) | 2 (7.7 %) | 5 (14.7 %) | 0.688 |
ICU: intensive care unit; VAP: ventilator associated pneumonia.
The median ICU length of stay was 26 (17) days. A longer ICU length of stay was found in the group change (26 [18]) versus the group maintenance (22 [16]), p = .051. Detailed analysis showed a successively decreasing trend from patients' subgroup in which directed antibiotherapy was initiated (32 [30]), antibiotherapy adjusted (28 [13]), de-escalation (24 [32]) and finally therapy was maintained (22 [16]), p = .266 (Table 3).
Length of ICU stay according to different therapeutic approaches (n = 60)
| Antibiotic therapy | Change (n = 26) | Maintenance (n = 34) | p | ||
| Length of ICU stay, (days) | 26 (18) | 22 (16) | 0.051 | ||
| Correction | De-escalation | Direct | |||
| 28 (13) | 24 (32) | 32 (30) | 22 (16) | 0.266 |
ICU: intensive care unit; VAP: ventilator associated pneumonia.
The main finding of this preliminary study was that in this population with suspected late VAP and prior antibiotherapy, positive BAL and quantitative cultures allowed changes in therapeutic approach, which were in concordance with those described in literature, resulting in antibiotic adequacy and consumption reduction. In addition, there were no statistically significant differences between patients submitted to different therapeutic approaches concerning ICU mortality or length of stay.
The BAL microbiological yield obtained was of 29.2 %. A meta-analysis of randomized studies 19 documented a microbiological positivity in patients submitted to invasive techniques that varies between 44.1 and 68.9 %. However, in these studies, besides the mean duration of IMV being lower, between 6 and 11 days compared with 13 days in our study (median value coincided with mean), also the patients percentage with prior antibiotic prescription was considerably lower, between 50.4 and 78.2 % versus 100 % in our patients. These differences are probably due to the fact that attending to resources concerns in our environment, BAL has been mainly used in late PAV in patients with signs of clinic deterioration, non response despite empirical therapy or immunocompromised, which may explain the higher percentage of prior antibiotherapy use or suspension impossibility, and, therefore, the higher probability of false negatives results.7
Agents isolated on BAL were consistent with those predicted in this type of nosocomial pneumonia. 20,21 According to Trouillet, VAP classification in four groups according to IMV duration and the presence or absence of antibiotherapy may be a useful strategy for antibiotic selection.20 Relevance is placed on the need for covering potentially MDRO, such as P. aeruginosa, A. baumannii, S. maltophilia and MRSA, particularly implicated in group 4 pneumonia (> 7 days of IMV and prior antibiotherapy), as was the case of those included in our study, in which these agents were recovery in 56.3 %.
After knowing BAL cultural result, therapeutic approach was changed in 38.0 %, a rate that is consistent with the described in the previously mentioned meta-analysis (27.0 to 41.7 %).19
In addition, and although a statistical difference was not found, BAL quantitative cultures seem to have influenced prescription, which was more frequent when CFU counts were > 104 (48.5 % versus 28.9 %). Of notice that therapeutic changes were also made with bacterial loads below the threshold of 10 4 (15.8 %), based on the understanding that patient's immunosuppression status and prior antibiotherapy should be considered in quantitative cultures interpretation.7
In most cases, empirical therapy was adequate. Inappropriation was responsible for antibiotic changes in 16.9 %. P. aeruginosa (57.1 %) was the most frequent agent responsible for a non suspected drug resistance. Aspergillus and Candida spp. (60.0 %), considered pathogenic in immunosuppression context, were the most often non suspected agents isolated.
De-escalation occurred in 12.7 %, both by reduction in antibiotics number and spectrum narrowing. However, de-escalation can also occur through reduction in the duration or discontinuation of antibiotherapy. With negative cultures and satisfactory evolution, antibiotics discontinuation is of particular importance,22 namely in the presence of non-fermentative Gram negative bacilli, where a negative result is significant, even under antibiotherapy or recent change of antibiotics, as these agents are frequently persistent and hard to eradicate.7 It is important to enhance, however, that as negative cultures were not analysed there may be an underestimation in the true percentage of de-escalation and therapeutic changes in these patients, so this is a limitation to point out.
It is also important to refer literature data documenting that de-escalation occurs more often when quantitative cultures of invasive techniques are used,14,17 as opposed to non-invasive, while others contradict these findings. 23 In contrast, it seems to be consensual that de-escalation occurs more frequently after implementation of antibiotherapy protocols, based on international recommendations and local epidemiology.24 Despite the mentioned, the high percentage of multiple site infections may affect de-escalation possibility, as occurred in patients in which therapy was maintained, in whom de-escalation based on quantitative cultures had only been possible in a small percentage of cases (9.1 %).
Finally, lower ICU overall mortality and length of stay were found in patients in whom therapy was maintained, corroborating that “right at first time“ may be associated with a better prognosis. On the other way, VAP attributable mortality was lower in the group in which therapy was changed after BAL result knowledge and although this group had a longer ICU length of stay, it was clearly influenced by the value obtained in patients in which direct antibiotherapy was started. Moreover, although the limited size of the sample in each group/subgroup imposes serious limitations in regard to the outcomes analysis, the absence of a statistically significant difference between patients submitted to different therapeutic approaches, may be in agreement to some literature data, indicating that invasive techniques could lead to prescription changes but have no impact on patients' outcomes.19
The impact of BAL use as compared with non invasive techniques can only be inferred by another type of study, namely a randomized one.
Conflict of interestAuthors declare that they don't have any conflict of interest.