Deep lymphatic drainage plays an important role in the lung, as it removes foreign materials laying on the airways surface, such as pathogenic microorganisms. This drainage is also associated with lung tumour dissemination route. Liposomes with a specially tailored membrane were used as foreign particles to be removed by the lung lymphatics. We aim to obtain images of deep lung lymphatics in baboons using liposomes encapsulating 99mTc-HMPAO, as aerosols. Axillary lymph nodes were visualized 30 min post-inhalation, becoming more evident 1 hour after, when abdominal aortic and inguinal lymph nodes were also observed. Late images added no additional information. ROI's and their time-activity curves were drawn to obtain biokinetic information. In conclusion, we can say that the proposed technique enables visualization of the deep lymphatic lung network and lymph nodes. This methodology may be an important tool for targeted lung delivery of cytotoxic drugs.
A drenagem linfática profunda desempenha um papel importante no pulmão, uma vez que remove materiais estranhos depositados sobre a superfície das vias respiratórias, tais como microrganismos patogénicos. Esta drenagem está igualmente associada às vias de disseminação tumoral. Liposomas com uma membrana especificamente desenhada foram usados para simular partículas estranhas a ser removidas pelos linfáticos pulmonares. Pretendem obter-se imagens dos linfáticos profundos em babuínos usando liposomas que encapsulam 99mTc-HMPAO sob a forma de aerossol. Observaram-se gânglios linfáticos axilares 30 min pós-inalação, que se tornaram mais evidentes 1 hora após, quando os gânglios abdominais e aórticos também se tornaram visíveis. Imagens tardias não acrescentaram informação relevante. Foram desenhadas ROI's (regiões de interesse), bem como as correspondentes curvas de actividade-tempo para obter informação acerca da biocinética. Em conclusão, pode dizer-se que a técnica proposta torna possível a visualização da rede linfática profunda do pulmão e os gânglios linfáticos. Esta metodología poderá vir a ser importante na libertação pulmonar controlada de fármacos citotóxicos.
The dense deep lymphatic network in the visceral pleural connective tissue, both in peribronchial and perivascular connective sheets, of all lung lobes, and in the juxta-alveolar regions plays a crucial role in removing foreign materials and in lung tumour dissemination.1–5
Liposomes have been proposed as promising anti-tumoral drug carriers, due to their encapsulating ability.6,7 They are also suitable for imaging the deep lymphatic network, as they will act as foreign particles to be drained. They can be administered by several routes such as aerosols. Based upon the pathophysiology of Bacillus tuberculosis pulmonary infection, specific liposomes can be modulated. They can be made to mimick the membrane composition of the Bacillus subtilis spores (a respiratory tract saprophyte microorganism) in order to be captured by pulmonary lymphatics.8,9
Materials and methodsChemicals1Distearoylphosphatidylcholine (DSPC) was selected as the main phospolipid (transition temperature 56°C), enabling the production of stable liposomes in presence of biological fluids.6,7,10 Phosphatidylglycerol (PG) was chosen as a negatively charge phospholipid11,12 and glutamic acid (GA) acts as a residue, being present in the internal layer and in the dense outer layer. 13,14 DSPC, PG, GA and GSH were obtained from Sigma (St. Louis, MO, USA), and Sephadex G-25 from Pharmacia (Upsala, Sweden). For anaesthesia, Ketalar® (Parke Davis, Cape Town, S.A.) and Sagatal (Kyron Laboratories Pty. Ltd., Benrose, S.A.) were used. 99mTc was obtained from a commercial 99Mo/99mTc generator (NECSA, South Africa). Exametazime (Ceretec™) was purchased from G.E. Healthcare (UK). For labelling efficiency and radiochemical purity determination, strips of ITLC-SG (Gelman Sciences Inc., Ann Arbor, USA) and Whatman no. 1 paper were used.
Liposome preparationThe designed liposomal formulation is composed of DSPC:PG:GA, respectively 8:1:1, in molar ratio. The lipidic mixture with a 50mg/mL concentration was dissolved in 2mL of chloroform in a round-bottom flask. Then it was evaporated at room temperature, under reduced pressure and inert atmosphere, for 2 hours, to form a thin lipid film, which was dried overnight in vacuum.15 100mM reduced GSH in 0.9 % saline was added to the films by energetic vortex mixing.16–19 The flask was placed in a water bath at 65°C for 10min, to hydrate the lipidic film.
The produced multilamellar liposomes were then extruded at 70°C through two stacked polycarbonate filters (Nucleopore, CA, USA) of 100nm pore size, mounted in a mini-extruder (LiposoFast™, Avestin, Canada) fitted with two 0.5mL Hamilton syringes (Hamilton, NV, USA).20–22 In order to obtain unilamellar liposomes, with small polydispersity index, they were passed through the filters 20 times.23
To remove any remaining extravesicular GSH, the vesicle suspension (500 μL at a time) was eluted through a Sephadex G-25 gel molecular exclusion chromatography minicolumn at room temperature, plugged with a Durapore® membrane filter (Millipore, Ireland) of 0.45μm pore size. 23–26 The columns were washed with 0.9 % saline, pH = 7.4, with a flow of ± 21mL/h.15,22,27
Labelling proceduresLiposome labelling was done according to Phillips et al. 18 Ceretec® kits, containing 0.5mg exametazime, 7.6μg SnCl2 and 4.5mg NaCl were reconstituted with 740MBq (20mCi) of 99mTc pertechnetate in 1mL of 0.9 % NaCl solution, and incubated for 5min.
Using a three step ITLC system, according to the manufacturer, the reconstituted kits were tested for contamination by free pertechnetate, reduced hydrolysed 99mTc and hydrophilic 99mTc-exametazime complex, as well as for lipophilic 99mTc- exametazime.28 Only kits with lipophilic 99mTc-exametazime > 80 % were used for liposome labelling.
Approximately 3mL of liposomal solution were mixed with 0.5mL of 99mTc-exametazine. After 10min of incubation, liposomes were separated of any free 99mTc using a sephadex G-25 column. Labelling efficiency of 99mTc-exametazime-liposomes was checked by ITLC-SG in 0.9 % saline. In this system, liposomes remain at the origin, while contaminants move with the solvent front.28–30
Liposome size and surface-charge measurementsLiposome surface charge was determined by laser Doppler velocitometry, using a Coulter Delsa 440 at 4 light incidence degrees: 34.7°, 26°, 17.4° and 8.7°. These data were used to calculate the electrophoretic mobility and zeta potential of the samples.
Vesicle size distribution was determined by dynamic light scattering or photon correlation spectroscopy analysis with a Coulter N4 Plus.31,32 The obtained diffusion coefficient was used to calculate the average hydrodynamic radius and, therefore, the mean diameter of the vesicles.32,33
Stability studiesLiposome membrane permeability was evaluated in vitro along time by ITLC-SG with 0.9 % saline. A decrease of labelling efficiency, i.e., loss of aqueous core content, can be used as an index of membrane integrity.
Liposome stability was evaluated by two methods: a) incubation at 37°C with: saline, human serum, human plasma and human serum albumin solution (4mg/mL), both fresh and after complement deactivation of blood fractions (56°C, 30min), being saline and human serum albumin solution the controls;6,34, and b) ITLC-SG with saline, each 30min, during 5.5 hours after a second sephadex G-25 gel molecular exclusion chromatography.
Effect of ultrasound (2.7MHz frequency) on the integrity of the liposome membrane was also studied. Liposomes were evaluated before and after 3min nebulization by microchromatography, using the previously described system.35–37
Aerosol production and administrationAerosols were produced using an ultrasonic nebulizer (Heyer Ultraschall Verebler 69, Germany), generating US (ultrasound) of 2.7MHz frequency2.
The obtained heterodispersed aerosol was administered directly into a intratracheal tube inserted in the baboons' trachea (4 adult males, 25–27kg), until about 2000 Kcounts/min in the total field of view of the gamma camera were recorded (± 3min). Animals were anaesthetised throughout the study. To induce anaesthesia Ketalar® (10mg/Kg, i.m.) was used, immediately followed by a controlled infusion of Sagatal® (25–30mg/kg at 30mL/h, i.v.).
The urinary bladder of all animals was catheterized throughout the study, to drain the urine and enabling better pelvic image acquisition.
The effectively inhaled radioactivity dose (74 to 148MBq) was determined using a calibrator (Capintec) and measuring the remaining labelled solution in the nebulizer after aerosolisation.
The protocol for the in vivo studies was approved by the Ethics Committee of the University of Pretoria, according to the guidelines of the national Code for Animal Use in Research, Education, Diagnosis and Testing of Drugs and Related Substances in South Africa.3
Biodistribution studiesBiodistribution studies were performed in four baboons (Papio ursinus) placed in dorsal decubitus, over the gamma camera (Siemens Orbiter, Siemens, Erlangen, Germany), following preliminary results obtained in Sus scrofa.38 For dynamic acquisition (64 × 64 matrix, 1 frame/min for 30min) the collimator was placed under the thorax. The acquisition was synchronized with 99mTc-exametazime-liposome inhalation. A series of static images (128 × 128 matrix, 2min/frame) of thorax and pelvis was acquired at 30, 60, 90 and 120min post-inhalation.
Indirect lymphoscintigraphy was done in one baboon, to confirm the inguinal lymph nodes localization. 18.5MBq of 99mTc- Re2S7 were injected into the first interdigital space of both feet to perform a 30min dynamic acquisition (64 × 64 matrix, 1 frame/min) of the pelvis. Immediately after a pelvic static image (128 × 128 matrix) was acquired, doing passive movements of both feet.
As a control, one baboon inhaled a 99mTc-exametazime aerosol. Dynamic acquisition followed the previously referred protocol, as well as the static images. These images were used as background for subtraction in the 99mTc-exametazime-liposome images.
ROIs were drawn over lung, heart, axillary lymph nodes, liver, kidney, and bladder dynamic images. In order to obtain biokinetic information, time-activity curves were plotted.
Statistical methodsData are reported as mean ± standard deviation. A t-Student analysis was applied to the means, being the accepted probability for a significant statistical difference p < .05.
ResultsThe used methodology enabled production of unilamellar vesicles (mean diameter 50–100nm) with a small polydispersity index (0.17, n = 3), and a surface charge of –45.8mV (n = 3), confirmed by zeta potential and by photon correlation spectroscopy determination.
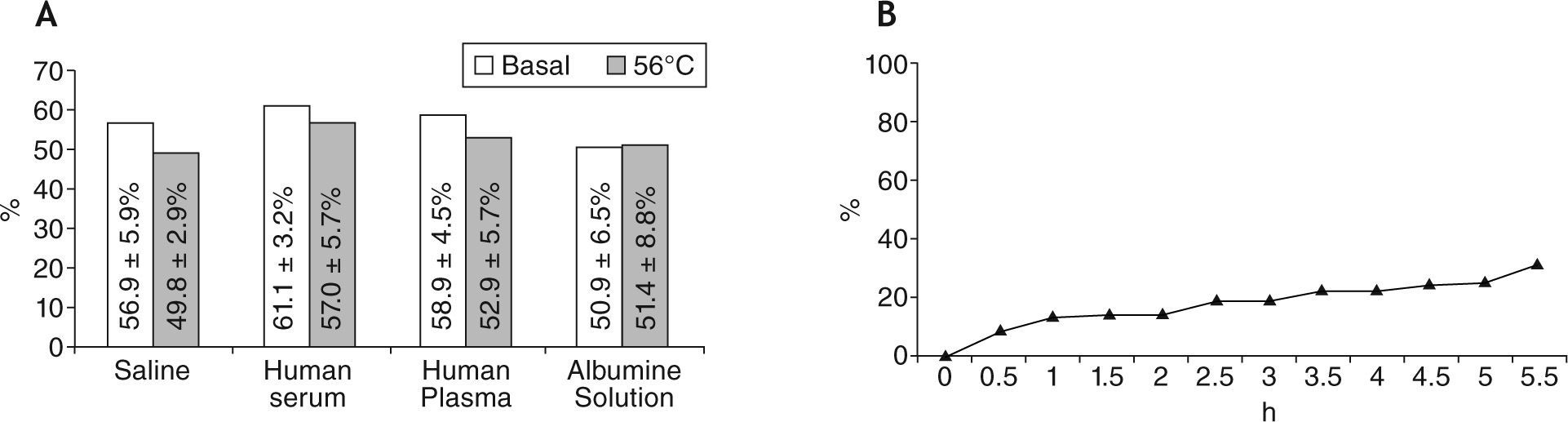
The labelling efficiency of the liposome formulation was tested by ascendant instant thin layer microchromatography (ITLC-SG) with saline. The average labelling efficiency obtained for 99mTc-exametazime was 74.1 ± 13.9 % (n = 12). The previously referred in vitro stability studies are shown in Figure 1Af. Labelling efficiency of 99mTc-exametazime-liposomes was good, but the most important feature was that in vitro stability in the presence of human serum, human plasma and albumin solution was higher than in the presence of blood fraction.
Stability of liposomes. A) Labelling efficiency (%) after incubation with different fluids (saline, human serum, human plasma, human serum albumin solution with a concentration of 4mg/mL), before and after warming at 56°C for 30min, in order to inactivate the complement of blood fractions. B) Temporal evaluation of the liposomes' aqueous core loss, using ascendant ITLC-SG with saline.
The liposomal formulation was administered as an aerosol, and its labelling efficiency (after 3min of US action) was not significantly different from the values determined pre- and post-US exposure. Study of liposomal aqueous core content loss, during 5.5 hours after the second ITLC, showed a good stability before and after ultrasonication (Fig. 1B).
Dynamic scintigraphic studies, both of 99mTc-exametazime and 99mTc-exametazime-liposomes aerosol inhalation, showed a good deposition in the lung, confirming that the produced liposomes reached the small airways and, hence, the alveolar surface.
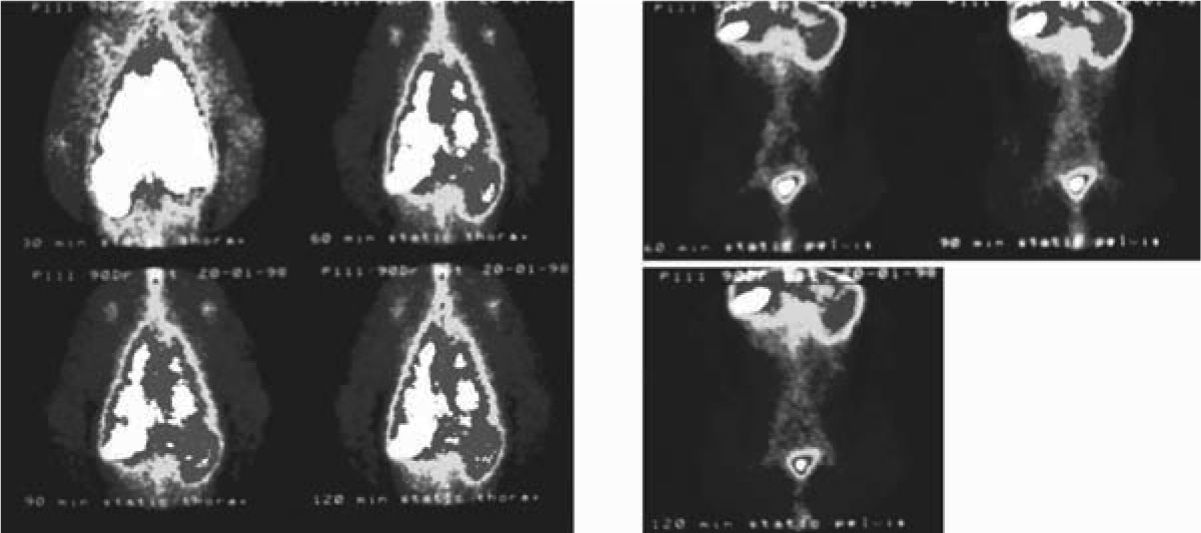
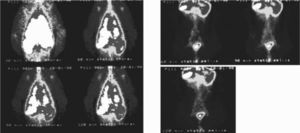
Axillary lymph nodes were visualized 30min post-inhalation. 1 hour post-inhalation they became more evident, and abdominal aortic and inguinal lymph nodes were also observed. Later images did not give additional information, although activity is observed in abdominal organs (Fig. 2).
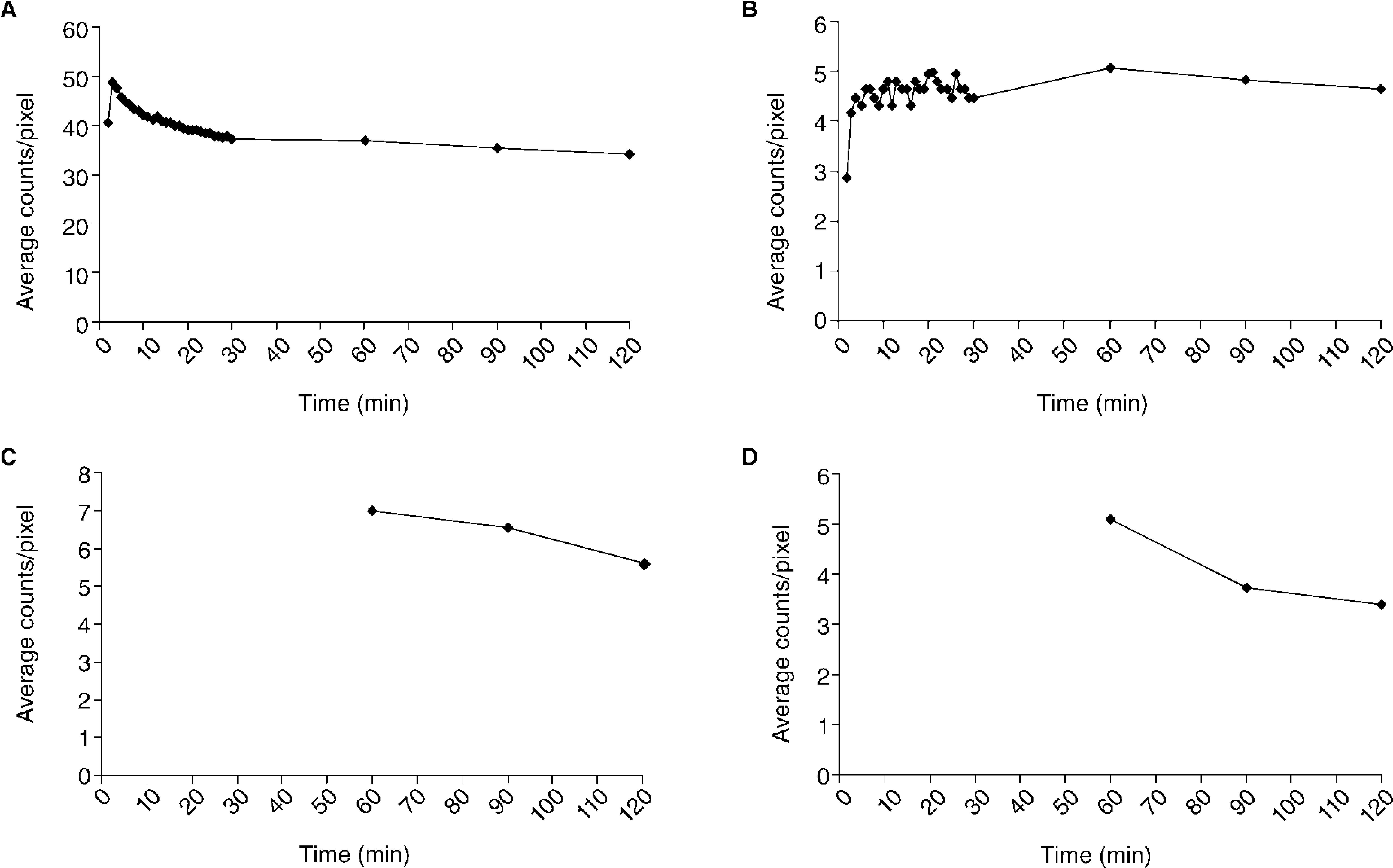
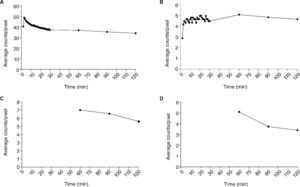
The mean activity/pixel in each ROI, after background and decay correction, was used to plot regional time-activity curves, showing the radiotracer biokinetics variations in certain target areas during the study (Fig. 3). Total activity was measured in the lungs post-inhalation, as well as clearance rate (t1/2 = 77min) (Fig. 3A). An increase of activity was simultaneously observed in axillary lymph nodes, reaching maximum activity values for axillary and inguinal nodes ± 60min (Fig. 3B-D).
Time-activity curves representing the kinetics of the 99mTc-Exametazime-liposomes in the lungs (A), axillary (B), periaortic (C) and inguinal (D) nodes. Graphics C and D begin only at 60min, since the collimator's dimension impairs to perform, simultaneously, a dynamic acquisition at thorax and abdominal levels.
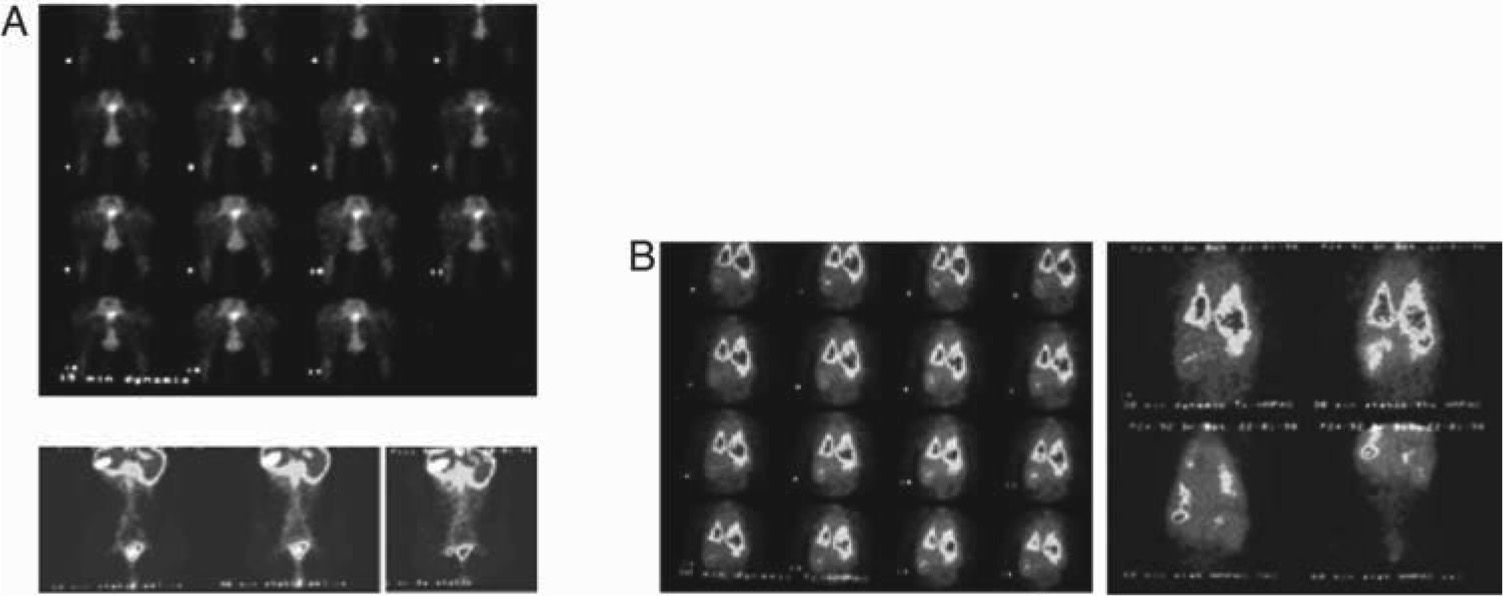
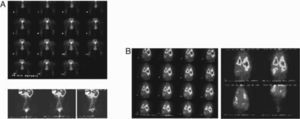
Promoting passive movement of the feet, after 99mTc-Re2S7 injection, the left inguinal node was visualized at 45min, corresponding to the area visualized with 99mTc-exametazime-liposomes (Fig. 4A). In a control animal, 99mTc-exametazime biodistribution showed a lung fast clearance through alveolar-capillary permeability, which enabled a quick visualization of several organs (liver, gallbladder, spleen, kidneys and the ascending and transverse colon (Product datasheet, Ceretec®, 2001). Nevertheless, lymph nodes were not evident; as abdominal activity masks the lymphatic abdominal chains (Fig. 4B).
Animal controls. A) Dynamic image sequence of the indirect lymphoscintigraphy obtained during the first 30min after interdigital feet 99mTc-Re2S7 injection, as well as static images at 60, 90 and 120min. The observed lymphatic abdominal drainage corresponds to the visualized areas with liposomes labelled with 99mTc-exametazime. B) 99mTc-exametazime biodistribution is a control and does not visualize the lymph nodes or lymphatic abdominal chains, only thoracic and abdominal activity uptake.
Following MIRD4 rules and applying the absorbed fraction method to calculate the absorbed dose, the obtained values are similar to those used in conventional nuclear medicine routine studies, to evaluate alveolar-capillary permeability with aerosols.39,40
DiscussionPulmonary lymphatic network is crucial for alveolar and interstitial clearance, being responsible for removal of many substances, particles, dusts, or pathogenic agents. Preferential lymphatic drainage correlates with certain specific surface components, such as those present in microorganisms.
This paper describes the obtained results of the in vivo animal studies in normal baboons, using the referred formulation (99mTc-exametazime-liposomes), administered as an aerosol as a diagnostic imaging agent to visualize the deep lung lymphatic drainage.
This carrier could be used both for visualization and therapy, whether it encapsulates an imaging agent or a therapeutic drug, respectively. Since anionic compounds are quickly removed by the lymphatic network negatively charged small calibrated unilamellar liposomes were produced by extrusion through polycarbonate membranes of 100nm pore size.41
Since 99mTc has 6 hours of half-life, labelling the liposome aqueous phase, after their formation, was done immediately prior to administration.7,11,18,23,24,34,42 Extrusion under moderate pressures was done, as liposome inhalation implied absence of organic solvents or detergents in the solution, to avoid possible allergic reactions.11
In vivo stability of liposomes may change and be altered by complement aggression, depending on the lipidic composition of the membrane.10 To evaluate these disruptive effects, 99mTc-exametazime-liposomes stability was tested in vitro, by incubation with saline, fresh human: serum, plasma and serum albumin solution (4mg/mL), as previously described. Results showed a statistically significant increase of labelling efficiency in presence of human serum and plasma for the studied formulation (t = 3.2; p = .01). This shows that biological fluids do not induce liposome aqueous content leakage. Stability to ultrasonication was determined analysing the labelling efficiency after 2.7MHz US action. Results do not show statistically significant differences for labelling efficiencies pre- and post-US action, as previously mentioned by other authors.37,41–45
Liposomal stability was also studied in terms of entrapped content loss (Fig. 1B), during 5.5 hours. Results showed a progressive increase of aqueous content loss, probably depending on the lipidic composition.
The inhaled thin liposomal aerosol duly reached the lung alveolar-capillary membrane. The vesicles could either be cleared by crossing the alveolar surface to lung interstitium after phagocytosis by alveolar macrophages, or directly through intercellular spaces or mucociliary escalator. Those, which crossed the interstitium, were eliminated by lymphatic drainage to the lymph nodes and subsequently to blood capillaries.46–48
Anatomically, pulmonary lymphatics can be grouped into two interconnected networks: the superficial pleural one, running in the connective tissue of the visceral pleura, and a deep intrapulmonary network forming the peribronchovascular lymphatics, located in the connective tissue sheets of the pulmonary bronchial and vascular trees. Several lymphatic capillaries can be seen in juxta-alveolar areas, in contiguity with the alveolar wall and separated from the alveolar lumen only by the alveolar epithelium and supporting connective tissue (usually very thin and richly vascularized). Pulmonary lymphatics can also be found in the loose connective tissue supporting more peripheral pleural cells and covering the pulmonary lobes, interalveolar septa and perivascular sheets.3–5,41,49
The clearance mechanism also depends on physicochemical characteristics and particle size. Only submicronic sizes (< 50nm Ø) can be deposited on the alveolar surface, being drained afterwards by the lymphatic system.50 After reaching the alveolar surface, the 99mTc-exametazime-liposomes cross into the lymphatic capillaries of juxta-alveolar areas through intercellular gaps and are engulfed by alveolar macrophages, migrating then to the hilar lymph nodes.3–5,41,49,51 In our animal model, the rather high radioactive dose deposited in the lungs, did not help to identify these nodes. Nevertheless, the main lymphatic drainage route of the whole organ is linked to the mediastinal and abdominal periaortic lymph nodes, therefore their visualization in the images agrees with the anatomical data (Fig. 2).1–5,41,49,51 There is a fast infra-abdominal lymphatic drainage, post-inhalation, confirmed by indirect lymphoscintigraphy. This clearance can possibly explain distant metastasis of lung cancer appearing in unexpected sites.
ConclusionsAerosols of specially tailored 99mTc-exametazime-liposomes proved to be an interesting approach to study deep lung lymphatic drainage. The physiological behaviour of these drug carriers, mimicking some properties of micro-organisms, allowed visualization of a descendant lymphatic pathway to the abdominal aortic chain nodes, confirmed by indirect lymphoscintigraphy. Images of these chains could give highly relevant information for staging lung tumours, as well as to evaluate other pathologies with important pulmonary lymphatic contribution. In addition, this methodology may play an important role in targeted lung delivery of other pharmaceuticals, e.g. cytotoxic drugs.
Taking into account the promising obtained results in the tested animal models, the production of a tracer, for inhaled administration, providing information on the degree of pulmonary invasion and metastization through functional images, is in perspective.
Conflict of interestAuthors declare that they don't have any conflict of interest.
All chemicals and reagents not specified in the text were of analytical grade or equivalent.
The water of the reservoir was cooled down to 5-6ºC in order to minimize the effects of temperature increase produced by the US.
- Home
- All contents
- About the journal
- Metrics
- Open access