The aim of this study was to determine patient-perceived characteristics of Chronic Obstructive Pulmonary Disease (COPD) in patients participating in a large trial evaluating tiotropium bromide.
Patients and methodsBaseline symptoms were assessed by means of a standardized questionnaire. Patients reported symptoms that led to diagnosis as well as their current most troublesome symptom.
ResultsData were obtained from 298 patients, mostly male (95 %), with mean (standard deviation) baseline forced expiratory volume in 1 second of 1.1 (0.4) L (40.6 [13.3] % of predicted), mean disease duration of 14.4 (10.1) years and smoking history of 55.1 (25.3) pack-years. Dyspnoea was the most frequently reported symptom leading to COPD diagnosis (55.0 % of patients), followed by cough (33.2 %). Dyspnoea was also the current most troublesome symptom (82.6 %), followed by cough (8.4 %). The presence of dyspnoea or cough was independent of COPD severity. The most commonly reported co-morbidities were cardiovascular disorders (49 % of patients), gastrointestinal disorders (20 %) and metabolic disorders (16 %), mainly diabetes mellitus.
ConclusionsThis analysis confirms the importance of dyspnoea as the most common symptom leading to initial COPD diagnosis and the symptom most troublesome to patients. Co-morbidities are common among COPD patients, and hence spirometric testing is appropriate in a patient who presents with dyspnoea associated with such a condition.
Este estudo teve como objectivo determinar os principais sintomas percepcionados pelos doentes com doença pulmonar obstrutiva crónica (DPOC) numa coorte de doentes que participaram num grande ensaio clínico, que avaliou o tiotrópio e que decorreu em Portugal.
População e métodosA caracterização dos sintomas, no momento de avaliação basal dos doentes foi efectuada através do recurso a um questionário padronizado. Os doentes foram inquiridos quanto aos principais sintomas que tinham levado ao diagnóstico e também quanto ao sintoma actual mais problemático.
ResultadosOs resultados foram obtidos de 298 doentes, maioritariamente masculinos (95 %), que apresentavam, uma média (desvio padrão) de volume expiratório forçado no primeiro segundo basal de 1,1 (0,4) L (40,6 [13,3] % do valor preditivo), uma duração média da doença de 14,4 (10,1) anos e uma carga tabágica de 55,1 (25,3) Unidades Maço Ano. A dispneia foi o sintoma mais frequentemente reportado, como tendo sido o sintoma que levou ao diagnóstico da doença (55,0 % de doentes), seguindo-se-lhe a tosse (33,2 %). A dispneia foi também o síntoma actual mais problemático (82,6 %), seguindo-se-lhe também a tosse (8,4 %). A presença de dispneia ou tosse foi independente da gravidade da DPOC. As comorbilidades mais frequentemente reportadas foram as doenças cardiovasculares (49 % dos doentes), gastrointestinais (20 %) e metabólicas (16 %), principalmente a diabetes mellitus.
ConclusõesEsta análise confirma a importância da dispneia como o sintoma mais comum que leva ao diagnóstico inicial da DPOC e o sintoma actual mais problemático para os doentes. As comorbilidades são comuns entre os doentes com DPOC, pelo que a espirometria deve ser realizada nos doentes que apresentem dispneia associada às patologias mais frequentes.
Chronic obstructive pulmonary disease (COPD) is increasingly recognized as a major public health challenge.1 COPD is characterised by reduced expiratory airflow that is commonly associated with symptoms of cough, sputum production and dyspnoea.2 Accurate symptom characterisation of patients in all stages of severity is an important step in diagnosis and treatment.
According to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines,3 diagnosis and severity of COPD is defined by lung function parameters. Despite a well-known clinical characterisation of the disease, more knowledge is needed about the natural history of symptoms and smoking status according to the different degrees of disease severity.
This study aimed to find out the main patient-perceived characteristics of COPD by disease severity that led to the diagnosis among patients participating in a clinical trial evaluating tiotropium bromide. The aim of this subanalysis was to identify the most frequent and most troublesome symptoms.
Patients and methodsStudy DesignThree hundred and eleven COPD patients were studied in a clinical trial evaluating tiotropium bromide, entitled SPIRIVA Assessment of Forced Expiratory volume in 1 second (SAFE; trial #205.282). This trial was a prospective, multicentre, randomized, double-blind, placebo-controlled parallel-group study designed to determine whether the effect on lung function of inhaled tiotropium 18g once daily over 12 weeks was affected by smoking status,4 where thirty-one Portuguese respiratory centres participated. The trial was approved by each local ethics committee and institutional review board. All patients provided written informed consent prior to the initiation of any study-related procedure.
The primary endpoint was change in trough forced expiratory volume in 1 second (FEV1) after 12 weeks of treatment.4
During the screening visit, evaluation of patients included the collection of each patient's demographic data, medical and smoking histories, concomitant diagnoses and therapies and a physical examination, including lung function tests.
The study groups consisted of outpatients of either sex who were aged ≥ 40 years and had a clinical diagnosis of COPD, as defined by the American Thoracic Society (ATS).5 Participants were required to have at least a 10 pack-year smoking history, clinically stable airway obstruction and moderate or severe COPD, as defined by a pre-bronchodilator FEV1 ≤ 70 % of predicted normal values and a FEV1/forced vital capacity (FVC) ≤ 70 %. This definition of COPD severity was according to the current GOLD guidelines, when the study was designed (2002),3 where pre-bronchodilator rather than post-bronchodilator FEV1 values were used. Patients were not included if they were considered to be at risk of COPD (defined as having normal lung function associated with cough and sputum production), again per the GOLD recommendations dated on 2002.3 Other exclusion criteria were a history of asthma, allergic rhinitis, atopy, recent history (6 months or less) of myocardial infarction, unstable arrhythmia or any clinically significant disease that might put the patient at risk because of study participation. Patients with ≥ 3 exacerbations of COPD in the preceding year or an exacerbation or lower respiratory tract infection within the 6 weeks prior to randomization were also excluded. Patients also should not have received prior treatment with tiotropium.
Symptom assessmentsIn this analysis, two COPD symptom measures were assessed: the first symptom that led to COPD diagnosis and the most current troublesome symptom.
Regarding the first symptom that led to COPD diagnosis, this was taken from patients' charts, where past clinical history was described.
The most troublesome symptom currently, including wheezing, shortness of breath (dyspnoea), cough and tightness of the chest, was evaluated by means of a standardized questionnaire at randomization (see online appendix for supplementary details). Each investigator completed this form, which provided an assessment of the patient's symptoms, based on the patient's condition during the week prior to giving the information of the most troublesome symptom. In this assessment, the severity of each symptom was rated as mild (awareness of symptom, which was easily tolerated), moderate (causing sufficient discomfort to cause interference with usual activity) or severe (incapacitating, with inability to work or perform usual activity). Symptom assessments were performed before spirometric testing.
Spirometric testingBaseline measurements were recorded between 07:00 and 10:00am, 2 weeks prior to randomization. Spirometric manoeuvres were conducted in triplicate and the highest FEV1 and FVC values were recorded. Measurements were performed in a Datospir 120C spirometer (Sibelmed, Barcelona, Spain) in accordance with ATS criteria.6 Predicted normal values for FEV1 and FVC were derived from standard equations.7
Statistical analysisData analysis was based on the patient cohort included in the primary study analysis. Oracle clinical was used to manage the data and statistical analysis was performed using SAS software (SAS Institute, version 8.2). A descriptive analysis of the patients according to the questionnaire was performed. Data are presented as mean and standard deviation (SD). Statistical comparisons used the Student t test for parametric data, Mann Whitney U tests for nonparametric data, and χ2 test for descriptive data. A P < 0.05 was considered significant.
ResultsAtotal of 311 patients from 31 study sites were randomized to treatment. A further 13 patients were excluded from the analysis after randomization due to the following reasons: 7 changed their smoking status during the study and 6 did not fulfil the inclusion criteria when reassessed at the baseline visit. Hence, 298 patients were included in the analysis according to severity at baseline (Table 1). Patients were mostly male and had moderate to very severe COPD, as defined by the GOLD stages but using pre-bronchodilator FEV1 values.
Demographics and baseline data
| Moderate | Severe | Very severe | Total | |
| No. of patients | 81 | 142 | 75 | 298 |
| Males (%) | 90 | 99 | 96 | 96 |
| Age (years)a | 64.5 (9.8) | 66.1 (8.3) | 63.2 (8.5) | 64.9 (8.9) |
| Duration of COPD (years)a | 13.4 (10.8) | 14.8 (10.4) | 13.7 (8.8) | 14.4 (10.1) |
| Smoking history (pack-years)a | 56.7 (25.1) | 54.0 (25.2) | 55.6 (26.0) | 55.1 (25.3) |
| Current smoker (%) | 23 (28.4) | 38 (26.8) | 14 (18.7) | 75 (25.2) |
| FEV1 (L)a | 1.6 (0.3) | 1.1 (0.2) | 0.7 (0.1) | 1.1 (0.4) |
| FEV1 (% predicted)a | 57.7 (6.4) | 39.3 (5.6) | 24.5 (4.0) | 40.6 (13.3) |
| FVC (L)a | 3.0 (0.7) | 2.5 (0.6) | 2.0 (0.5) | 2.5 (0.7) |
| FEV1/FVC (%)a | 53.2 (9.2) | 44.6 (9.4) | 36.6 (9.5) | 44.9 (11.1) |
| BMI (kg/m2)a | 27.3 (5.1) | 26.9 (5.2) | 24.4 (4.4) | 26.4 (5.1) |
| Visits to emergency room in previous 12 monthsa | 0.5 (1.2) | 0.6 (2.3) | 1.1 (3.0) | 0.7 (2.3) |
| Courses of antibiotic | 0.84 (0.98) | 1.01 (1.21) | 1.25 (1.56) | 1.02 (1.26) |
| Courses of steroids | 0.33 (0.94) | 0.39 (0.83) | 0.85 (2.51) | 0.49 (1.47) |
BMI: body mass index; COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity.
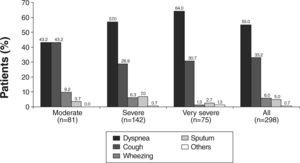
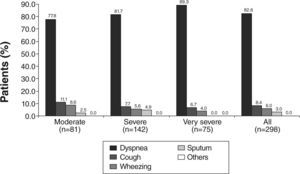
Dyspnoea was the most frequently reported symptom that led to COPD diagnosis in 55.0 % of patients. It was also the most frequently reported symptom leading to diagnosis in every disease stage, except in the moderate stage, where dyspnoea and cough were equally prevalent (Fig. 1). With regards to the current most troublesome symptom reported by patients across all subgroups of severity, dyspnoea was also the most commonly reported symptom, being reported by 82.6 % of patients (Fig. 2). Cough was the second most frequently reported symptom leading to diagnosis (in 33.2 % of patients) (Fig. 1) and also the second currently most troublesome symptom (8.4 %) (Fig. 2). In the moderate disease severity group, the frequency of cough and dyspnoea was equal at the time of diagnosis, as referred above (Fig. 1). However, when compared with dyspnoea, cough had an inverse frequency pattern (Fig. 2), if disease severity is taken into account. It is worth noting that only 5.0 % of patients reported sputum production as the first symptom that led to the diagnosis (Fig. 1).
Concerning the severity of symptoms at baseline, dyspnoea was mild or moderate in 75.9 % of patients; cough was mild or moderate in 64.3 % of patients and wheezing in 53.0 % of patients (Table 2).
There was no statistically significant association between smoking status (current versus past smokers) and the first symptom leading to diagnosis (P = .699) or to the most troublesome symptom (P = .554).
Similarly, there was also no statistically significant association between body mass index (BMI) and the first symptom leading to diagnosis (P = .80) or to the most troublesome symptom (P = .336).
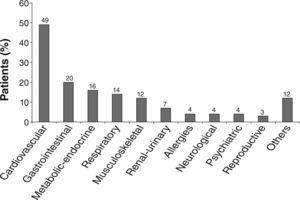
Almost half (49 %) of the patients enrolled in the study had a cardiovascular condition in the last 5 years, and this was the most frequent concomitant diagnosis (co-morbidity), followed by gastrointestinal disease (20 %) and metabolic-endocrine conditions (16 %) (Fig. 3). Among the group of cardiovascular, arterial hypertension was the most frequent, being present in 88 % of the global group of patients. In the gastrointestinal disease group, 85 % had peptic disease, and in the metabolic disorders group 53 % had diabetes mellitus. Concomitant pulmonary disorders were reported in 14 % of patients (n = 48). Of these 14 had diagnosis of obstructive sleep apnea syndrome or past tuberculosis (n = 8), and 26 had a diagnosis that could be considered a COPD complication: pneumonia (n = 13), COPD exacerbations (n = 4), pneumothorax (n = 4), bronchiectasis (n = 3), pulmonary thromboembolism (n = 1) and lung neoplasm (n = 1).
In the year prior to the study, the use of health care resources was ≥ 1 visit only in the very severe group, compared with the moderate and severe groups, as assessed by the number of visits to the emergency room (Table 1). Similarly, the very severe group tended to receive a greater number of prescribed antibiotic or oral steroid courses during the previous year than either of the other 2 severity groups.
DiscussionThis analysis of this study population demonstrates that dyspnoea was the most frequently reported symptom leading to the diagnosis of COPD at all stages of the disease and was also the most troublesome symptom at all levels of severity. These findings are consistent with previous experience that dyspnoea is usually the first symptom that causes a patient to seek medical attention. 8 By contrast, although cough is usually the first symptom to develop, it is often overlooked by patients.8 The reports of dyspnoea increased with increasing disease severity. These results suggest that dyspnoea should be considered as a diagnostic marker of COPD, even at less severe or moderate stages. As dyspnoea was the most troublesome symptom for patients with COPD, it should also be a primary treatment outcome.
It should be noted that cough, the main symptom leading to diagnosis in patients with moderate COPD, became a relatively less troublesome symptom with increasing disease severity, in contrast with dyspnoea, which increased in all severity groups.
Similar findings have been reported in the literature. A telephone survey of patients with COPD living in North America and in Europe documented the frequency of breathlessness with daily activities.9 According to this survey, one-fifth of patients reported that they were breathless, even when just sitting or lying still, and 24 % when talking. One-third said they were breathless when doing light housework or while getting washed or dressed, and nearly 70 % were short of breath when walking up a flight of stairs. It is clear from these data that COPD is associated with a considerable burden of disease, affecting many activities that are fundamental to everyday life, such as the ability to breathe, talk, work, sleep, have sexual activity and socialise. However, in the same study, 9 COPD patients also regarded their disease as mild to moderate, despite suffering from relatively severe dyspnoea. This latter finding highlights the difficulties inherent in diagnosing COPD on symptomatic grounds. The condition is often under-diagnosed, at least partly because patients do not recognise relevant symptoms. 10 The diagnosis of COPD should be considered in any patient who presents with cough, dyspnoea or sputum production, particularly if the patient has been exposed to risk factors. 8,11 The best established risk factor for COPD is exposure to tobacco smoke;8 epidemiological studies have shown that, with sufficient exposure, most smokers will eventually develop airflow limitation.12 Hence, a positive smoking history was an inclusion criterion in the present study. Other risk factors include indoor air pollution, occupational exposure to dust, gases or fumes and genetic influences.8
The patients participating in this study had moderate or severe COPD, and were being treated in specialised centres. This raises the question as to the extent the findings are generalisable to the general population. In fact, because the patients were selected from specialised pulmonology services, they correspond to a subgroup of COPD patients and are not completely representative of the real life COPD patients. Accordingly, the extremely high prevalence of males (96 %) may result from this selection.
Akamatsu et al have reported that symptoms alone have a low sensitivity for the diagnosis of COPD, and recommended that the diagnosis be confirmed by spirometry. 13 Other studies, however, have shown that the use of symptom-based questionnaires to diagnose COPD is feasible in the general population. In one study, a questionnaire based on age, symptoms (wheezing and sputum production), smoking history, BMI and previous diagnosis of obstructive lung disease achieved a sensitivity of 85 % and a specificity of 45 %;10 this performance was comparable with that of other instruments designed for use in a general population.14 In our study, dyspnoea was the principal symptom leading to a diagnosis of COPD. By contrast, in a general practice study in Netherlands, chronic cough had the strongest predictive value for COPD among smokers. 15 However, it should be noted that, in this study, from a population of 169 smokers only 30 had airways obstruction; in addition, the obstruction criterion was FEV1 < 80 % of predicted normal values, allowing the inclusion of patients with less severe disease than in the current study (FEV, ≤ 70 % of predicted normal values).
Despite dyspnoea and cough being good predictors of COPD, current guidelines recommend that spirometry is needed to confirm the diagnosis in patients in whom COPD is suspected on symptomatic grounds and encourage the use of spirometry in primary care.3,11
Somehow unexpected was the finding of only 5 % of patients reported sputum production as the first symptom that led to COPD diagnosis. One explanation for this result could be memory bias, taking into account that mean disease duration at the time of evaluation was 14.4 years.
Another issue in our study is the large group of co-morbidities associated with COPD. It is possible that, in some cases these may have introduced confounding effect by producing symptoms that overlap with those of COPD. In particular, respiratory disorders were reported in 14 % of patients, and in the majority of the cases these could be considered complications of COPD. However, this interpretation requires caution. Although pneumonia is a common feature of COPD exacerbations, there are important differences between exacerbations with and without pneumonia; patients with pneumonia tend to show a faster onset of symptoms, and more severe illness, than those without.16,17 Although dyspnoea could be a symptom of congestive heart failure as well as COPD, it seems unlikely that this was a major confounding factor in this study because only 19 patients (6.4 %) had diagnoses of heart failure.
There is extensive evidence that co-morbidities are common among patients with COPD, although the reported prevalence vary widely. 17–26 There is, for example, a growing body of epidemiological evidence linking COPD with cardiovascular disease.21,22 Large population-based studies suggest that patients with COPD have 2 to 3 times the risk for cardiovascular mortality compared with controls.21–24 The profile of co-morbidities seen in our study was consistent with that reported in other studies.17,18,26 Moreover, considering that some exclusion criteria were related to the presence of some co-morbidity, the reported co-morbidities may underestimate their real occurrence. In accordance with our results, Antonelli Incalzi et al,25 in a study of 270 COPD patients discharged from hospital after an acute exacerbation of COPD, found that the most common co-morbid conditions were hypertension (28 %), diabetes mellitus (14 %) and ischemic heart disease (10 %). Also, in a review of the literature, Chatila et al17 reported that cardiovascular disorders are present in 13–65 % of COPD patients, hypertension in 18–52 %, diabetes mellitus in 5–16 % and gastrointestinal disorders in 15–62 %. More recently Agusti et al,26 in the description of the Eclipse cohort, reported higher prevalence of co-morbidities in COPD patients than in controls and with similar frequencies to our data.
This increased prevalence of co-morbidities in COPD may provide an opportunity to efficiently identify persons at risk for undiagnosed COPD and to get them appropriately evaluated, diagnosed and treated.
The findings of the present study highlight the importance of the association between dyspnoea and co-morbidities when considering a diagnosis of COPD. The authors suggest that, mainly in the primary care setting and internal medicine outpatient clinics where patients presenting with dyspnoea associated with co-morbid conditions such as cardiovascular disease, peptic disease and/or diabetes mellitus, are frequent, the diagnosis of COPD should be considered. As a consequence, a spirometry should be done in order to exclude a diagnosis of COPD.
Support statementThe study SAFE was funded by Boehringer Ingelheim (Lisbon, Portugal) and Pfizer (Lisbon, Portugal).
Conflict of interestAuthors declare that the don't have any conflict of interest.
The authors wish to acknowledge the following investigators for their contributions to the study: Dr. Maria de La Salete Valente, Dr. Ricardo Nascimento, Dr. Carlos Boavida, Dr. Ulisses Brito, Dr. Conceicao Antunes, Dr. Joao Roque Dias, Dr. Dias Pereira, Dr. Teresa Cardoso, Dr. Reis Ferreira, Dr. Jorge Roldao Viera, Dr. Abílio Reis, Dr. Aida Coelho, Dr. Sousa Barros, Dr. Carlos Alves, Dr. Amaral Marques PhD, Dr. Paula Simao, Dr. José Vieira, Dr. Bugalho de Almeida PhD, Dr. Olga Freitas, Dr. Paula Duarte, Dr. Joao de Almeida, Dr. Mariano Machado, Dr. Luís Goes, Dr. Simoes Torres, Dr. Maria Manuel Machado, Dr. Júlio Gomes and Dr. Luís Oliveira.
The authors also wish to acknowledge Dr. José Antunes, Dr. Marisa Sousa, Dr. Ana Cristina Bastos, Dr. Maria Luisa Santos, Esmeralda Violas and Conceicao Peralta for their work in support of this study. We also acknowledge the editorial support of Natalie Barker from PAREXEL MMS Europe Ltd, whose work was funded jointly by Boehringer Ingelheim and Pfizer.
Dr. Cristina Bárbara, PhD, Dr. Joaquim Moita, Dr. Joao Cardoso and Dr. Rui Costa were the top recruiters of this study. Dr. Raquel Redondeiro and Dr. Márcia Gaspar are employees of Boehringer Ingelheim. This study was funded by Boehringer Ingelheim and Pfizer.