Patients with diaphragmatic weakness complain of dyspnoea when lying supine, bending forward, swimming, or bathing. The corresponding mechanisms remain unclear. Septating the coelomic cavity, the diaphragm protects the lungs from abdominal compression.1 Diaphragm paralysis and atrophy abolish this protection. This reduces lung volume, particularly during postural changes because the transferred abdominal weight exerts an expiratory action on the unprotected lungs1. Lung compliance and upper airway collapsibility depend on diaphragm activity and their postural deterioration could contribute to orthopnoea through increased work of breathing.2,3 In addition, through the associated lung volume reduction, diaphragm dysfunction could lead to expiratory flow limitation (inability to increase expiratory flow when expiratory driving pressure augments). Expiratory flow limitation is associated with dyspnoea because the associated changes in operating lung volumes increase the inspiratory muscles burden.4 Postural expiratory flow limitation is associated with orthopnoea in chronic obstructive pulmonary disease (COPD), left heart failure (LHF), and morbid obesity.4,5 We therefore hypothesised that diaphragm weakness could be associated with a postural reduction in operating lung volume (reduction in expiratory reserve) hence expiratory flow limitation and orthopnoea.
The study was approved by the institutional review board of the French society for respiratory medicine (decision “CEPRO 2022-017”, April 2, 2022). Patients provided written consent. Inclusion criteria were: age over 18; diaphragm weakness (oesophageal pressure twitch response to bilateral phrenic stimulation —Poes,tw—< 11 cmH2O-6). Non-inclusion criteria were: obstructive ventilatory defect; body mass index over 30 kg.m−2; abdominal parietal defects. Dyspnoea was evaluated using a 10-cm visual analogue scale (VAS; 0, no difficulty; 10, worst imaginable). Orthopnoea was defined by an increase in VAS (∆-VAS) of 1 cm or more after lying supine. Sitting and supine vital capacity (VC), inspiratory capacity (IC), and expiratory reserve volume (ERV) were obtained using standard spirometry. Expiratory flow limitation was looked for by comparing resting breathing flow-volume loops (natural FVL) with FVLs in response to an increase in the alveolar-atmospheric gradient (stimulated FVL) determined by: 1) the application of a 5 cmH2O negative expiratory pressure at the mouth (Medisoft Expair®, Belgium); 2) manual abdominal compression.7,8 Expiratory flow limitation was identified when the stimulated FVL was partially or entirely superimposed on the natural FVL. Data are summarised as median [min-max]. Patients with and without expiratory flow limitation were compared using Mann-Whitney U test and Fischer's exact text. Statistical significance was set at p <0.05.
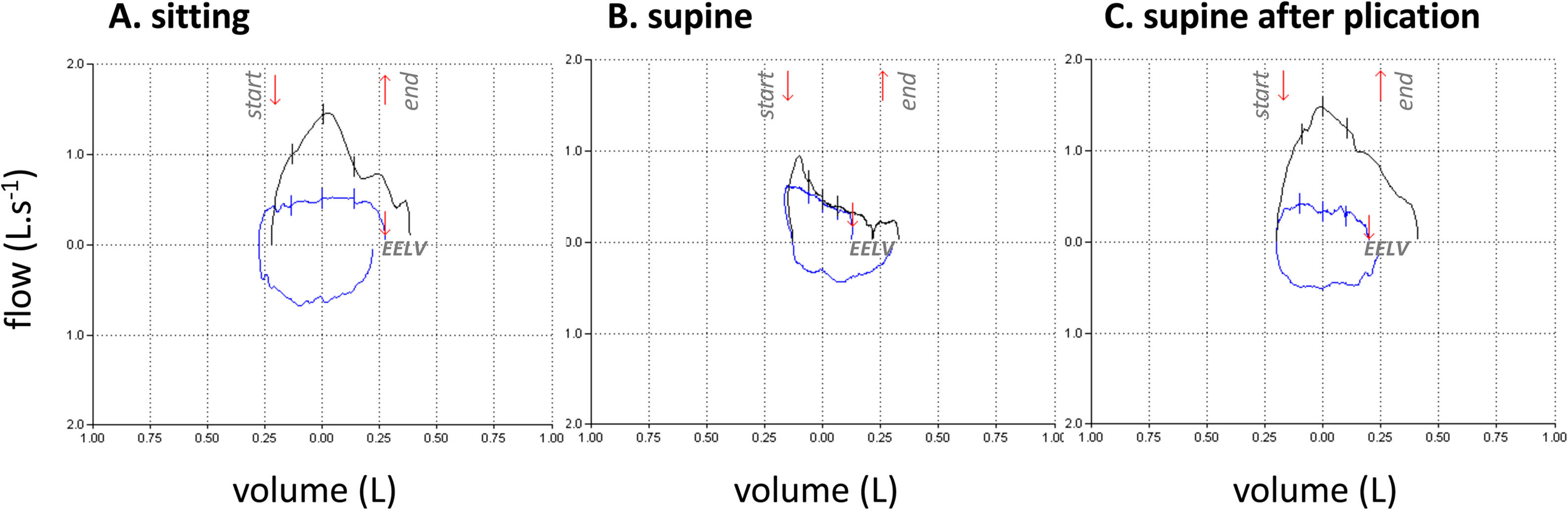
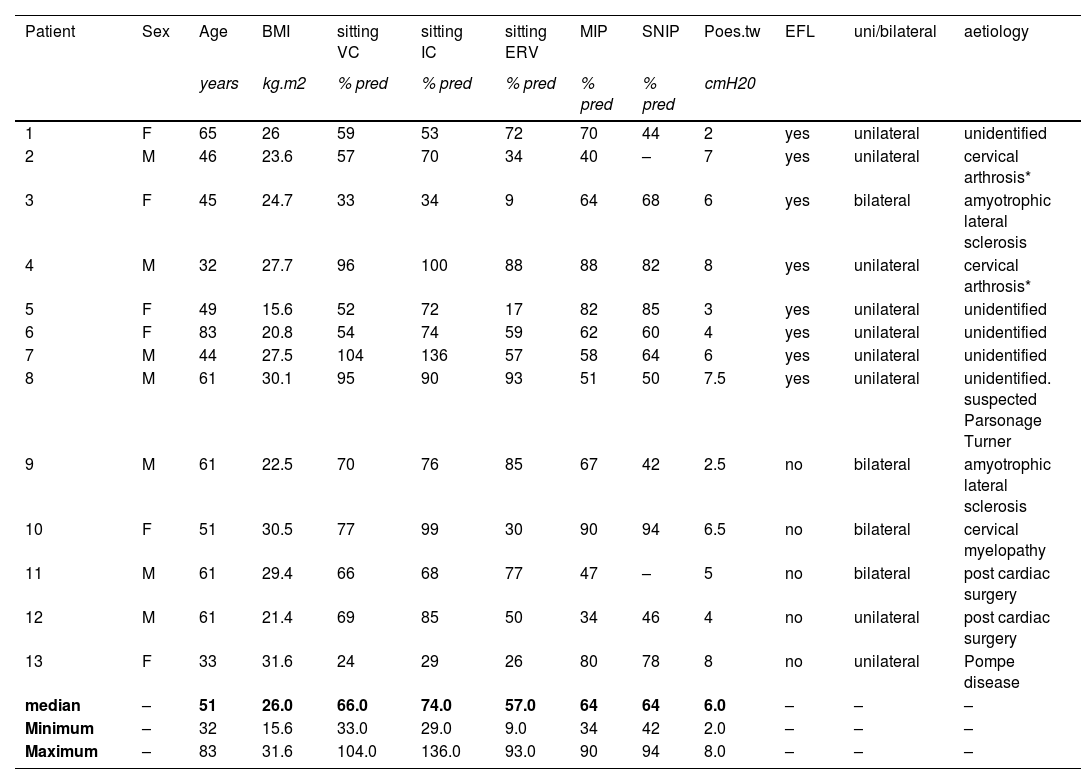
Thirteen patients were studied. Their characteristics are described in Table 1. ERV markedly decreased when supine (−35% [−22;−70]), the fall exceeding 20% in all patients. Supine changes in VC were modest and inconsistent (- 8% [−3; −32], falls exceeding 15% in only four patients and 20% in only two). The same was true for IC (1% [−0.21; +0.19]). Supine expiratory flow limitation occurred in 8 cases (example in Fig. 1A and 1B; perfect concordance between negative expiratory pressure and manual abdominal compression). These 8 patients experienced orthopnoea (∆VAS=3. [1,6; 6,8]). None of the remaining five patients had expiratory flow limitation, but one reported orthopnoea. The association of expiratory flow limitation with orthopnoea was statistically significant (p = 0.007). There was no discernible difference between uni- or bilateral diaphragm dysfunction. Two patients with expiratory flow limitation were studied three months after diaphragm plication: VC and ERV improved by about 15%, no supine fall in ERV were observed anymore, and both orthopnoea and expiratory flow limitation had disappeared (Fig. 1C).
Characteristics of the patients.
| Patient | Sex | Age | BMI | sitting VC | sitting IC | sitting ERV | MIP | SNIP | Poes.tw | EFL | uni/bilateral | aetiology |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| years | kg.m2 | % pred | % pred | % pred | % pred | % pred | cmH20 | |||||
| 1 | F | 65 | 26 | 59 | 53 | 72 | 70 | 44 | 2 | yes | unilateral | unidentified |
| 2 | M | 46 | 23.6 | 57 | 70 | 34 | 40 | – | 7 | yes | unilateral | cervical arthrosis* |
| 3 | F | 45 | 24.7 | 33 | 34 | 9 | 64 | 68 | 6 | yes | bilateral | amyotrophic lateral sclerosis |
| 4 | M | 32 | 27.7 | 96 | 100 | 88 | 88 | 82 | 8 | yes | unilateral | cervical arthrosis* |
| 5 | F | 49 | 15.6 | 52 | 72 | 17 | 82 | 85 | 3 | yes | unilateral | unidentified |
| 6 | F | 83 | 20.8 | 54 | 74 | 59 | 62 | 60 | 4 | yes | unilateral | unidentified |
| 7 | M | 44 | 27.5 | 104 | 136 | 57 | 58 | 64 | 6 | yes | unilateral | unidentified |
| 8 | M | 61 | 30.1 | 95 | 90 | 93 | 51 | 50 | 7.5 | yes | unilateral | unidentified. suspected Parsonage Turner |
| 9 | M | 61 | 22.5 | 70 | 76 | 85 | 67 | 42 | 2.5 | no | bilateral | amyotrophic lateral sclerosis |
| 10 | F | 51 | 30.5 | 77 | 99 | 30 | 90 | 94 | 6.5 | no | bilateral | cervical myelopathy |
| 11 | M | 61 | 29.4 | 66 | 68 | 77 | 47 | – | 5 | no | bilateral | post cardiac surgery |
| 12 | M | 61 | 21.4 | 69 | 85 | 50 | 34 | 46 | 4 | no | unilateral | post cardiac surgery |
| 13 | F | 33 | 31.6 | 24 | 29 | 26 | 80 | 78 | 8 | no | unilateral | Pompe disease |
| median | – | 51 | 26.0 | 66.0 | 74.0 | 57.0 | 64 | 64 | 6.0 | – | – | – |
| Minimum | – | 32 | 15.6 | 33.0 | 29.0 | 9.0 | 34 | 42 | 2.0 | – | – | – |
| Maximum | – | 83 | 31.6 | 104.0 | 136.0 | 93.0 | 90 | 94 | 8.0 | – | – | – |
BMI. body mass index; VC. vital capacity; IC. inspiratory capacity; ERV. expiratory reserve volume; MIP. maximal inspiratory pressure.
SNIP. sniff nasal inspiratory pressure; Poes.tw. twitch oesophageal pressure; EFL. expiratory flow limitation.
NB: room air pulsed transcutaneous haemoglobin oxygen saturation ≥ 95% il all cases.
Flow-volume curves recorded during tidal breathing (blue lines; inspiration bottom, expiration top) and in response to manual abdominal compression (black lines) in one typical patient, in the sitting posture (A), the supine posture (B), and the supine posture three months after surgical diaphragm plication (C). The red arrows at the top of the panels denote the start and the end of the abdominal compression. The red arrows superimposed on the tidal flow volume curves indicate end-expiratory lung volume (EELV). Panel B shows expiratory flow limitation, with the manual compression-induced expiratory flow not exceeding the spontaneous expiratory flow during most of the expiration. Expiratory flow limitation does not occur in A and C. Note that the shape of the tidal flow-volume curve is different in B, with a marked downward expiratory slope that is not seen in A and C.
As hypothesised, lying supine was consistently associated with a marked ERV decrease, attesting to an expiratory effect of the posture-related cephalad shift of the abdominal content in patients with diaphragmatic weakness. Expiratory flow limitation did not occur consistently, but its occurrence bore a statistically significant relationship with orthopnoea. We acknowledge that this is a small exploratory study and that we did not engage in a comprehensive pathophysiological dissection of the mechanisms of orthopnoea. Nevertheless, from our observations we propose that postural expiratory flow limitation contributes to diaphragm weakness-related orthopnoea. This closely resembles LHF, COPD, and massive obesity.4,5 In the two patients with diaphragm plication, the supine fall in ERV disappeared as did expiratory flow limitation and orthopnoea (Fig. 1C). In conclusion, and of clinical relevance, our data justify further investigation of the diagnostic value of postural changes in ERV in to screen for diaphragm weakness and its comparison with the more traditional postural changes in VC that are not very sensitive. Our data also justify further investigation of the value of postural expiratory flow limitation when discussing diaphragm plication, an intervention of which the indications are not codified and the results difficult to anticipate: the possibility of predicting the symptomatic results of diaphragm plication would also be of clinical relevance.
Data management and sharingAll data will be made available by the authors upon reasonable request.
Declaration of generative AI in scientific writingNo generative AI was used for the present manuscript.
Previous presentation at research meetingsNone.
Authors' contributionsStefania REDOLFI: conception and design; data acquisition and analysis; drafting; final approval; Vincent NINANE: conception and design; data analysis; final approval; Christian STRAUS: conception and design; data analysis; final approval; Thomas SIMILOWSKI conception and design; data acquisition and analysis; drafting; final approval.
FundingThis research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.