Linezolid (LZD) is highly effective and recommended by the WHO for treatment of multidrug-resistant tuberculosis (MDR-TB) including extensively drug-resistant tuberculosis (XDR-TB) despite its association to frequent adverse events (AEs).1 Very few studies investigated the factors associated with hematological and non-hematological LZD-related AEs in MDR/XDR-TB patients. We estimated the prevalence of LZD-related AEs and identified clinical characteristics associated with LZD-related AEs among LZD-treated patients based on data collected as part of a WHO active Drug Safety Monitoring observational study. This study was conducted in 45 centers in 26 countries between July 2017 and August 2019 by the Global Tuberculosis Network . The detailed methods of data collection are reported elsewhere.2 Adverse events were considered LZD-related if LZD was identified as the drug responsible by the site investigator.2 Myelosupression was defined as either anemia, leukopenia or thrombocytopenia.2 Information about the dosing (600–1200 md/day) was only available for patients with reported AEs.
From 658 patients’ data available, 518 patients receiving LZD-containing regimen were included in this study (Table 1). The total number of AEs was 446, majority were mild (grade 1–2). Among total number of AEs, the proportion of LZD-related peripheral neuropathy and optic neuritis were 9.9% and 1.6%, respectively. The proportion with LZD-related hematological toxicity of anemia, eosinophilia, bone marrow suppression, and thrombocytopenia were 5.4%, 3.6%, 0.9% and 0.7% of all AEs, respectively. Among 518 patients received LZD-containing regimen, 209 (40.3%) patients experienced at least 1 AEs; one-third of them (or 13.1% of total population) had LZD-related AEs. Permanent discontinuation of at least one drug because of AEs occurred in 114/518, (22.0%) patients. Among 68 patients experienced with LZD-related AEs, 28 patients (41.2%) had hematological AEs. Only 1 patient had an increase in lactate – which was only Grade 1–2 AE
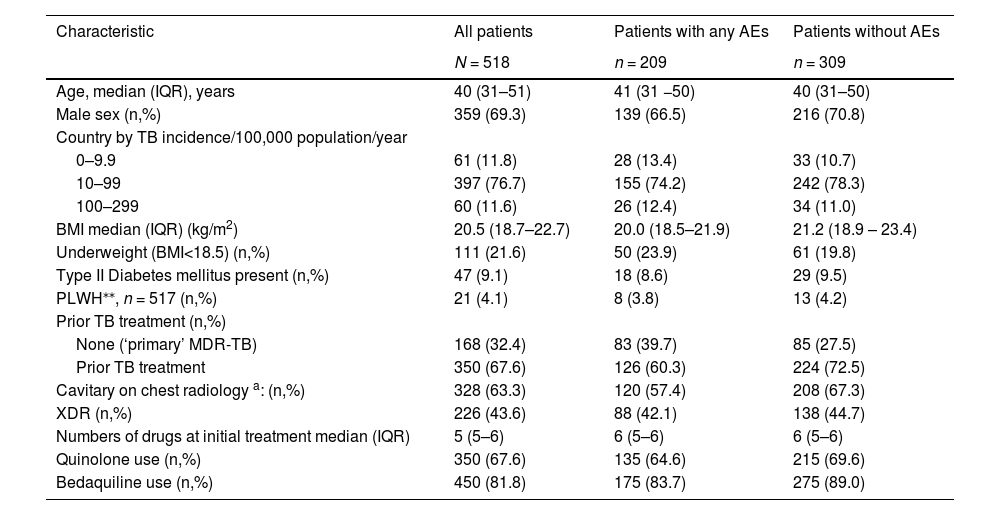
Clinical characteristics of patients who received LZD containing regimen, and developed any Adverse Events*, or none.
Patient and treatment characteristics were summarized using frequencies and percentages for categorical variables, and median and interquartile ranges (IQRs) for continuous. We calculated LZD-related AEs prevalence as the number of patients with at least one LZD-related AEs divided by the number of patients receiving at least one dose of LZD.
| Characteristic | All patients | Patients with any AEs | Patients without AEs |
|---|---|---|---|
| N = 518 | n = 209 | n = 309 | |
| Age, median (IQR), years | 40 (31–51) | 41 (31 −50) | 40 (31–50) |
| Male sex (n,%) | 359 (69.3) | 139 (66.5) | 216 (70.8) |
| Country by TB incidence/100,000 population/year | |||
| 0–9.9 | 61 (11.8) | 28 (13.4) | 33 (10.7) |
| 10–99 | 397 (76.7) | 155 (74.2) | 242 (78.3) |
| 100–299 | 60 (11.6) | 26 (12.4) | 34 (11.0) |
| BMI median (IQR) (kg/m2) | 20.5 (18.7–22.7) | 20.0 (18.5–21.9) | 21.2 (18.9 – 23.4) |
| Underweight (BMI<18.5) (n,%) | 111 (21.6) | 50 (23.9) | 61 (19.8) |
| Type II Diabetes mellitus present (n,%) | 47 (9.1) | 18 (8.6) | 29 (9.5) |
| PLWH⁎⁎, n = 517 (n,%) | 21 (4.1) | 8 (3.8) | 13 (4.2) |
| Prior TB treatment (n,%) | |||
| None (‘primary’ MDR-TB) | 168 (32.4) | 83 (39.7) | 85 (27.5) |
| Prior TB treatment | 350 (67.6) | 126 (60.3) | 224 (72.5) |
| Cavitary on chest radiology a: (n,%) | 328 (63.3) | 120 (57.4) | 208 (67.3) |
| XDR (n,%) | 226 (43.6) | 88 (42.1) | 138 (44.7) |
| Numbers of drugs at initial treatment median (IQR) | 5 (5–6) | 6 (5–6) | 6 (5–6) |
| Quinolone use (n,%) | 350 (67.6) | 135 (64.6) | 215 (69.6) |
| Bedaquiline use (n,%) | 450 (81.8) | 175 (83.7) | 275 (89.0) |
Adverse events were defined as any untoward medical occurrence in a patient administered a pharmaceutical product documented as related to specific drugs and graded according to Common Terminology Criteria for Adverse Events version 5.0. AEs was considered to be LZD related if this was identified as the drug responsible by the attending physician according to WHO Active TB Drug-Safety Monitoring and Management 2015.
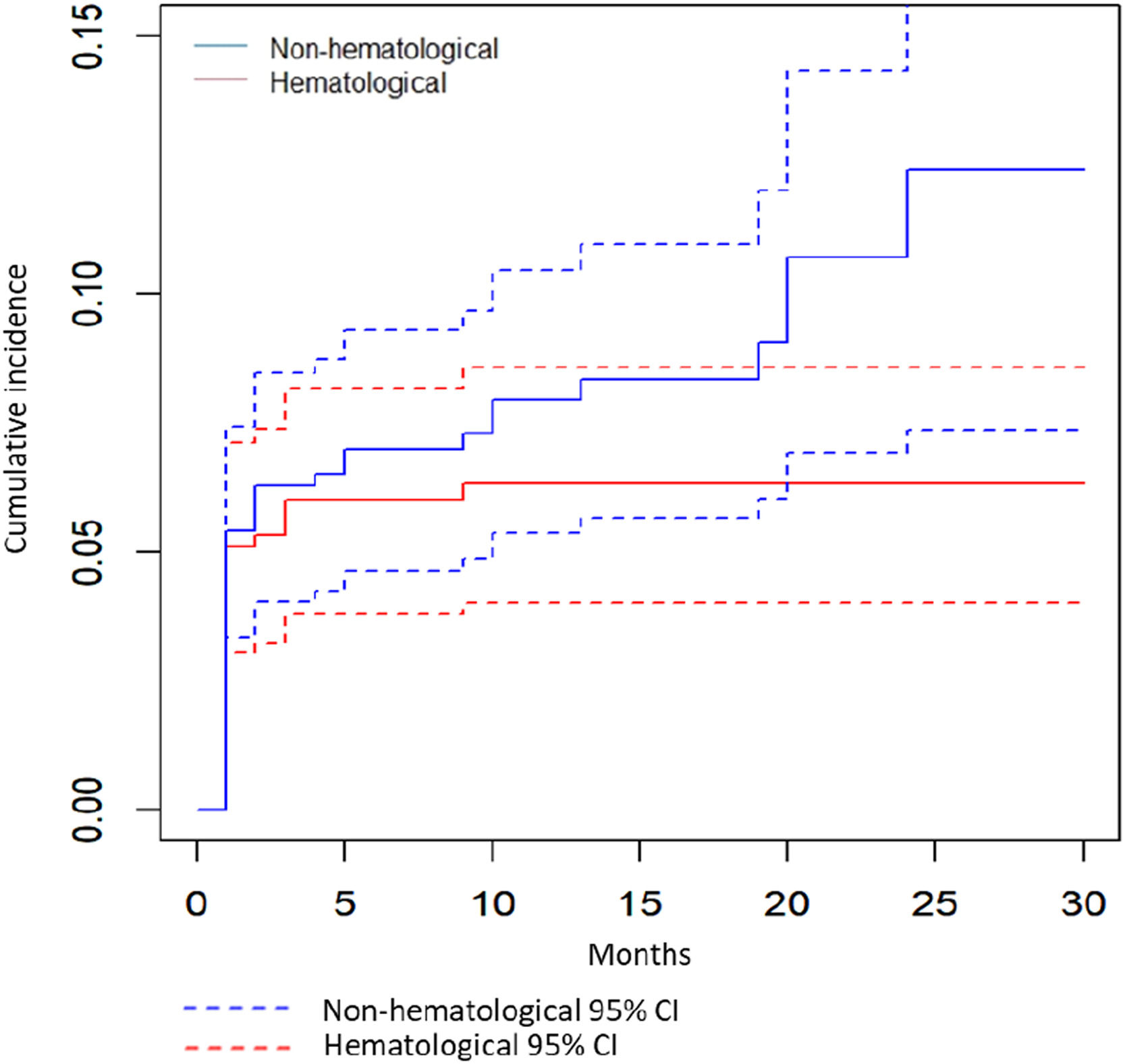
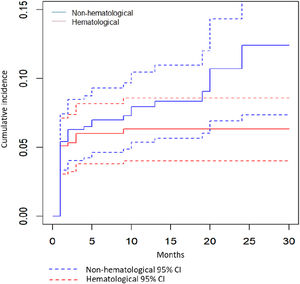
As seen in Fig. 1, the cumulative incidence of AEs increased rapidly during the first 6 months of treatment (Fig. 1). These AEs resulted in LZD dose change or discontinuation in about one fourth (132/518, 25.5%) of all LZD treated patients.
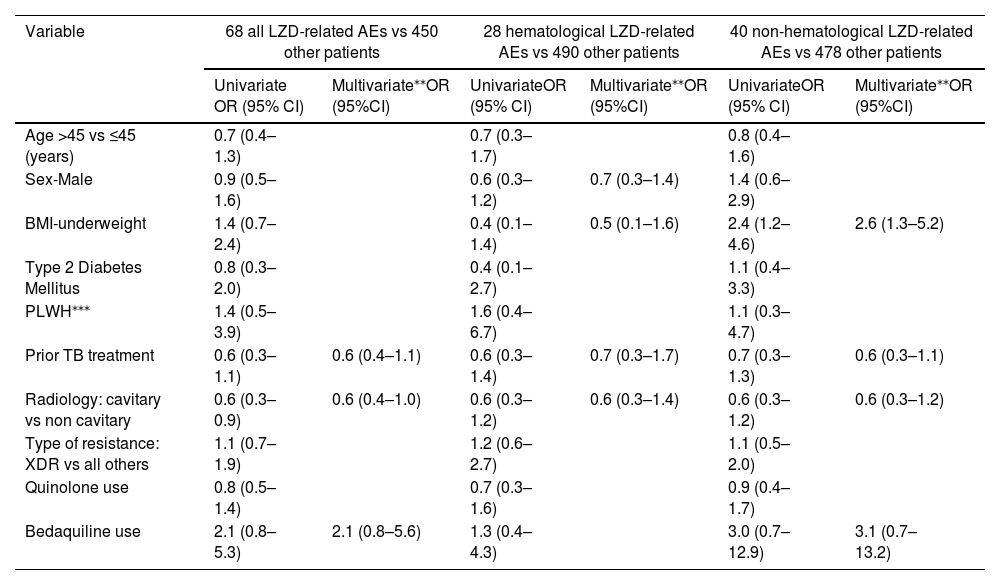
Univariate and multivariate analyses were conducted to show whether prior TB treatment, cavitation on chest radiology, and bedaquiline use were associated with LZD-related AEs (Table 2). Patients with BMI <18.5 were 2.6 times more likely to have non-hematological LZD-related AEs. No factors were significantly associated with hematological LZD-related AEs. Patients with any AEs had lower BMI compared to patients without AEs [20.0 (18.5–21.9 vs 21.2 (18.9 – 23.4), p 0.01, respectively]. This finding was consistent with study by Zhang et al., in which patients with lower body weight and BMI, had reduced clearance of LZD, and increased concentration of the drug in the blood and likelihood of AEs.3
Clinical characteristics associated with LZD-related Adverse Events* in 518 patients who received LZD. Multivariate logistic regression analysis was used to identify characteristics significantly associated with LZD-related AEs. The variables with p<0.25 in univariate analyses were included in multivariate logistic regression models. All analyses performed with R software, version 4.1.0, and the additional freely available R software packages. Kaplan Meier analysis was used to calculate cumulative incidence for LZD-related AEs.
| Variable | 68 all LZD-related AEs vs 450 other patients | 28 hematological LZD-related AEs vs 490 other patients | 40 non-hematological LZD-related AEs vs 478 other patients | |||
|---|---|---|---|---|---|---|
| Univariate OR (95% CI) | Multivariate⁎⁎OR (95%CI) | UnivariateOR (95% CI) | Multivariate⁎⁎OR (95%CI) | UnivariateOR (95% CI) | Multivariate⁎⁎OR (95%CI) | |
| Age >45 vs ≤45 (years) | 0.7 (0.4–1.3) | 0.7 (0.3–1.7) | 0.8 (0.4–1.6) | |||
| Sex-Male | 0.9 (0.5–1.6) | 0.6 (0.3–1.2) | 0.7 (0.3–1.4) | 1.4 (0.6–2.9) | ||
| BMI-underweight | 1.4 (0.7–2.4) | 0.4 (0.1–1.4) | 0.5 (0.1–1.6) | 2.4 (1.2–4.6) | 2.6 (1.3–5.2) | |
| Type 2 Diabetes Mellitus | 0.8 (0.3–2.0) | 0.4 (0.1–2.7) | 1.1 (0.4–3.3) | |||
| PLWH⁎⁎⁎ | 1.4 (0.5–3.9) | 1.6 (0.4–6.7) | 1.1 (0.3–4.7) | |||
| Prior TB treatment | 0.6 (0.3–1.1) | 0.6 (0.4–1.1) | 0.6 (0.3–1.4) | 0.7 (0.3–1.7) | 0.7 (0.3–1.3) | 0.6 (0.3–1.1) |
| Radiology: cavitary vs non cavitary | 0.6 (0.3–0.9) | 0.6 (0.4–1.0) | 0.6 (0.3–1.2) | 0.6 (0.3–1.4) | 0.6 (0.3–1.2) | 0.6 (0.3–1.2) |
| Type of resistance: XDR vs all others | 1.1 (0.7–1.9) | 1.2 (0.6–2.7) | 1.1 (0.5–2.0) | |||
| Quinolone use | 0.8 (0.5–1.4) | 0.7 (0.3–1.6) | 0.9 (0.4–1.7) | |||
| Bedaquiline use | 2.1 (0.8–5.3) | 2.1 (0.8–5.6) | 1.3 (0.4–4.3) | 3.0 (0.7–12.9) | 3.1 (0.7–13.2) | |
LZD dose is not included in this analysis because only stated in 68 patients with LZD-related AEs.
Adverse events were defined as any untoward medical occurrence in a patient administered a pharmaceutical product documented as related to specific drugs and graded according to Common Terminology Criteria for Adverse Events version 5.0. AEs was considered to be LZD related if this was identified as the drug responsible by the attending physician according to WHO Active TB Drug-Safety Monitoring and Management 2015. Hematological AEs included: anemia, thrombocytopenia, eosinophilia or bone marrow suppression.
In this study, the occurrence of AEs in LZD-treated patients was 40.3%. Any LZD-related AEs occurred in 13.1% patients, and hematological AEs occurred in 5.4%. The occurrence of AEs in LZD-treated patients was consistent with previous studies that reported AEs in 41%−82% patients treated with LZD-containing regimen.4 The prevalence of hematological LZD-related AEs in our study was substantially lower than in a previous study by Pratama et al. that found the hematological AEs in 27/93 (29.0%) of patients with MDR-TB who were treated with LZD; anemia was much more common than thrombocytopenia or leukopenia.5 Myelosuppression was reported in 7/38 (18%) XDR-TB patients treated with linezolid-containing regimen in Korea and 7/27 (25.9%) in South Africa.6 This discrepancy might be explained by the different method of reporting hematological AEs. Our study only categorized LZD-related hematological AEs based on the report in database.
The prevalence of non-hematological LZD-related AEs (mostly peripheral neuropathy) was lower than a previous study that reported peripheral neuropathy in 11/58 (19%) and optic neuritis in 1/58 (2%) MDR-TB patients treated with LZD in a retrospective study conducted at two TB reference hospitals in Netherlands and Italy.7 This variance might be explained by different LZD doses. Most of our patients (37/40, 92.5%) who experienced non-hematological AEs received LZD 600 mg/d, cumulative LZD dose and the number of days patients were exposed to LZD were significantly higher in the patients who experienced peripheral neuropathy. Discontinuation of LZD in a quarter of our patients because of AEs was consistent with previous studies.
We found that underweight patients (BMI <18.5) were 2.6 times more likely to experience non-hematological LZD-related AEs. To the best of our knowledge there was no study addressing this factor in MDR-TB treated with LZD. Underweight patient may have had nutritional deficiencies, most notably vitamin B that had been associated with peripheral neuropathy and are more commonly seen in individuals with all types of TB.
Our study had limitations. The non-randomized allocation of patients to LZD treatment could cause underestimating the risk of LZD-related AEs. A significant number of patients had not completed treatment, which could result in an underestimation of LZD-related AEs. The initial dose of LZD was only recorded for patients who experienced LZD-related AEs, preventing the evaluation of LZD dosage as a risk factor for AEs. Our study has notable strengths, including participation from multiple countries, a large sample size, and the possibility of describing the time to AEs occurrence. The study findings indicate that risk factors for hematological and non-hematological LZD-related AEs might differ.
In conclusion, more than 10% MDR-TB patients treated with LZD-containing regimen experienced LZD-related AEs. Hematological AES accounted for one third of all LZD-related AEs. Being underweight should be considered as a potential risk factor for non-hematological LZD-related AEs. Future studies should focus on identification of risk factors for LZD-related AEs, including dose and possible genetic factors.