Bronchiectasis is a common chronic respiratory disorder characterized by irreversible bronchial dilatation leading to daily productive cough and recurrent respiratory infections.1,2 This is a simplistic description of a multidimensional disease with heterogeneous clinical course and significant co-morbidity.3,4 According to expert opinion, along with vast evidence in other chronic diseases [e.g. chronic obstructive pulmonary disease (COPD) and asthma], accurate severity assessment is essential to guide decision-making treatment and disease management.5,6
Historically evaluated based on computed tomography (CT) features,7 bronchiectasis severity assessment has been increasingly recognized as an integration of many clinical, functional, radiological and microbiological factors.8,9 In the last few years, two multidimensional scoring systems, the Bronchiectasis Severity Index (BSI) and FACED have been developed to integrate those fundamental factors predicting prognosis of patients with bronchiectasis.10,11
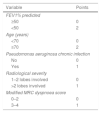
BSI was derived from a prospective cohort including 608 patients from United Kingdom (UK) and externally validated in independent cohorts from UK (n=344), Belgium (n=253) and Italy (n=105).10 FACED was developed using data from a retrospective cohort of 819 patients from Spain.11 Both scores comprise similar variables like age, Medical Research Council (MRC) dyspnoea score, forced expiratory volume in first second (FEV1) % predicted, Pseudomonas aeruginosa chronic infection and radiological severity. The major difference is that BSI also incorporates body mass index (BMI), hospitalizations in the past 2 years, exacerbations in the past 12 months, and chronic infection with bacteria other than P. aeruginosa (Tables 1 and 2). BSI awards different point values for each variable and FACED uses dichotomized variables with distinct cut-off points. Both scores classify bronchiectasis as mild (BSI score 0–4, FACED score 0–2), moderate (BSI score 5–8, FACED score 3–4) or severe (BSI score ≥9, FACED score 5–7). The scores are shown in Table 1.
The Bronchiectasis Severity Index.
| Variable | Points |
|---|---|
| Age (years) | |
| <50 | 0 |
| 50–69 | 2 |
| 70–79 | 4 |
| ≥80 | 6 |
| Body mass index (kg/m2) | |
| <18.5 | 2 |
| ≥18.5 | 0 |
| FEV1% predicted | |
| >80 | 0 |
| 50–80 | 1 |
| 30–49 | 2 |
| <30 | 3 |
| Hospitalizations in the past 2 years | |
| No | 0 |
| Yes | 5 |
| Exacerbation frequency in the past 12 months | |
| 0–2 | 0 |
| ≥3 | 2 |
| MRC dyspnoea score | |
| 1–3 | 0 |
| 4 | 2 |
| 5 | 3 |
| Bacterial chronic infection | |
| Pseudomonas aeruginosa | 3 |
| Other potentially pathogenic microorganisms | 1 |
| None | 0 |
| Radiological severity | |
| ≥3 lobes involved or cystic bronchiectasis | 1 |
| <3 lobes involved | 0 |
Since their development, some studies have tried to compare and contrast the evaluation of disease severity and the prognostic value of these two scoring systems. In the last edition of the Pulmonology, Costa et al.12 explored this issue. They performed a retrospective study of 40 patients from Coimbra, Portugal to compare classification of bronchiectasis severity between BSI and FACED. Three important findings should be stressed. First, about one third of patients were classified in each severity risk category based on BSI. In contrast, according to FACED half of patients had mild bronchiectasis, and only 12.5% had severe bronchiectasis. Second, more than three quarters of patients classified as severe using BSI had mild or moderate disease by FACED, and almost half of patients with moderate disease according to FACED had severe disease by BSI.
The key conclusion of the work by Costa et al., in our opinion, is that BSI and FACED may contain similar variables, but are clearly measuring very different things because there is limited correlation between them.12
Previous studies have shown similar results. The largest study in this field that included 1612 patients from seven European cohorts participating in the European Bronchiectasis registry project showed that BSI scored most patients as moderate or severe (mean 6.0–9.7), while mild disease predominated according to FACED (mean 1.5–2.3).13 In a single centre cohort study by Ellis et al.14 enrolling 74 patients, BSI identified 31% of patients as severe versus 8% with FACED. Moreover, 19 patients considered severe by BSI had a mild or moderate FACED score. More recently, Rosales-Mayor et al.15 described that, in a prospective cohort of 182 patients, severe disease accounts for 54% of all patients with bronchiectasis based on BSI, while the majority (59%) of patients were classified as mild or moderate by FACED.
So what are we measuring? And for whom?
The data so far suggests that, as shown by Costa and colleagues, the two scores measure different things. FACED was designed to predict mortality – a job it does very well.13–15 Most patients with bronchiectasis are at low risk of 5-year mortality because unlike idiopathic pulmonary fibrosis or lung cancer, bronchiectasis is not a rapidly fatal disease. As a result, most patients with bronchiectasis are classified as “mild” or “moderate” by FACED.11–15
This is appropriate when considering the risk of mortality, but what effect might this have on clinical practice if misinterpreted? A 50 year old patient having 5 exacerbations per year, frequent hospitalizations and chronically infected with methicillin resistant Staphylococcus aureus but with FEV1 >50% predicted for example, will score as “mild” according to FACED, but no reasonable clinician would regard their burden of disease as “mild”. FACED is relatively simple but its simplicity is also its weakness. A score of 5 or more points out of 7 is required to classify a patient as severe, with 2 points each awarded for age >70 and FEV1 <50% predicted. This leads to unfortunate conclusions such as, for example, no patient under 70 can have severe bronchiectasis unless they also have P. aeruginosa chronic infection.11 It has been shown by a number of authors that “mild, moderate and severe” patients according to FACED have a similar frequency of exacerbations, similar levels of quality of life impairment and similar symptoms making FACED an inappropriate tool to measure burden of disease.10–15
So in our opinion, where FACED is concerned it is more accurate to talk about patients being at low, moderate and high risk of death and not about “mild”, “moderate” or “severe”.
Exacerbations are the key clinical end-points in bronchiectasis clinical trials and are a major driver of morbidity and mortality.16–20 We argue it is not possible to talk about severity of bronchiectasis without talking about exacerbations. A key question to ask all patients with bronchiectasis is about the number and severity of exacerbations they have experienced. The European Respiratory Society guidelines based a number of their recommendations, including those for long-term antibiotic treatment on the history of exacerbations.21
Therefore the greatest strength of the BSI is that it places a large degree of weight on the history of exacerbations. It also suffers from the limitation of including age as a factor, and is relatively complex. The complexity is reduced by the ready availability of an online calculator at www.bronchiectasisseverity.com. It is much more effective at predicting patients at high risk of future exacerbations, worse quality of life and patients with more severe symptoms compared to FACED.10–15
So how should clinicians evaluate severity of disease in daily practice for clinical decision making?
Examples of major decisions include when to use inhaled or oral prophylactic antibiotics, how often to monitor patients and when to refer for additional intervention such as surgery or lung transplant assessment.21
In the authors’ opinion, the consistency of the “frequent exacerbator” phenotype in bronchiectasis is such that, the history of exacerbations should be the major deciding factor in clinical practice for the use of therapies aimed at reducing exacerbations, such as macrolides and inhaled antibiotics.22 The combination of frequent exacerbations plus P. aeruginosa chronic infection, for example, is associated with poor clinical outcomes across all spectra – mortality, hospitalizations, quality of life and future exacerbations. Irrespective of scoring therefore, patients should be considered for antimicrobial treatment in accordance with ERS guidelines.8,9
The rigorously evidence-based ERS guidelines for bronchiectasis published in 2017 do not recommend using severity tools in making any treatment decisions, suggesting instead to use a threshold of 3 or more exacerbations per year to guide long-term antibiotic treatment (for example).21 They do, however, suggest using the BSI alongside other factors such as co-morbidities and the severity of quality of life impairment to lower this threshold in some cases. The logic of this approach is that a patient with a higher BSI score and 2 exacerbations per year is at high risk of future exacerbations and complications including hospital admission and so may benefit from prophylactic treatment. FACED is not mentioned in the ERS guidelines as none of the current treatments available for bronchiectasis reduce mortality, and therefore it is not possible to recommend treatment, monitoring of referral based on mortality scoring.
In summary, comparing BSI and FACED has clearly established that they measure two different things. BSI is a severity assessment tool, while FACED accurately predicts risk of death. Clinicians should use the appropriate tool for the appropriate context, depending on what they wish to predict and why.
It is now more important than ever to increase awareness of adverse prognostic features in bronchiectasis to increase the overall quality of treatment and reduce the devastating burden of the disease.21,22