To identify predictors of immune-related adverse events (IRAEs) in patients with non-small cell lung cancer (NSCLC) treated with immune checkpoint inhibitors (ICIs). Assess associations between outcomes and the development of IRAEs.
MethodsRetrospective analysis of patients with NSCLC treated with ICIs between 2016 and 2020 in the Pulmonology Department of our hospital. Patients with and without IRAEs were compared. A logistic regression analysis was performed to determine predictors of IRAEs. Progression-free survival (PFS) and overall survival (OS) curves were calculated using the Kaplan-Meier method, and the long-rank test was used to assess survival differences between groups. Univariate and multivariate Cox proportional-hazards regression models were used to identify factors associated with PFS and OS. The value considered statistically significant was p≤0.05.
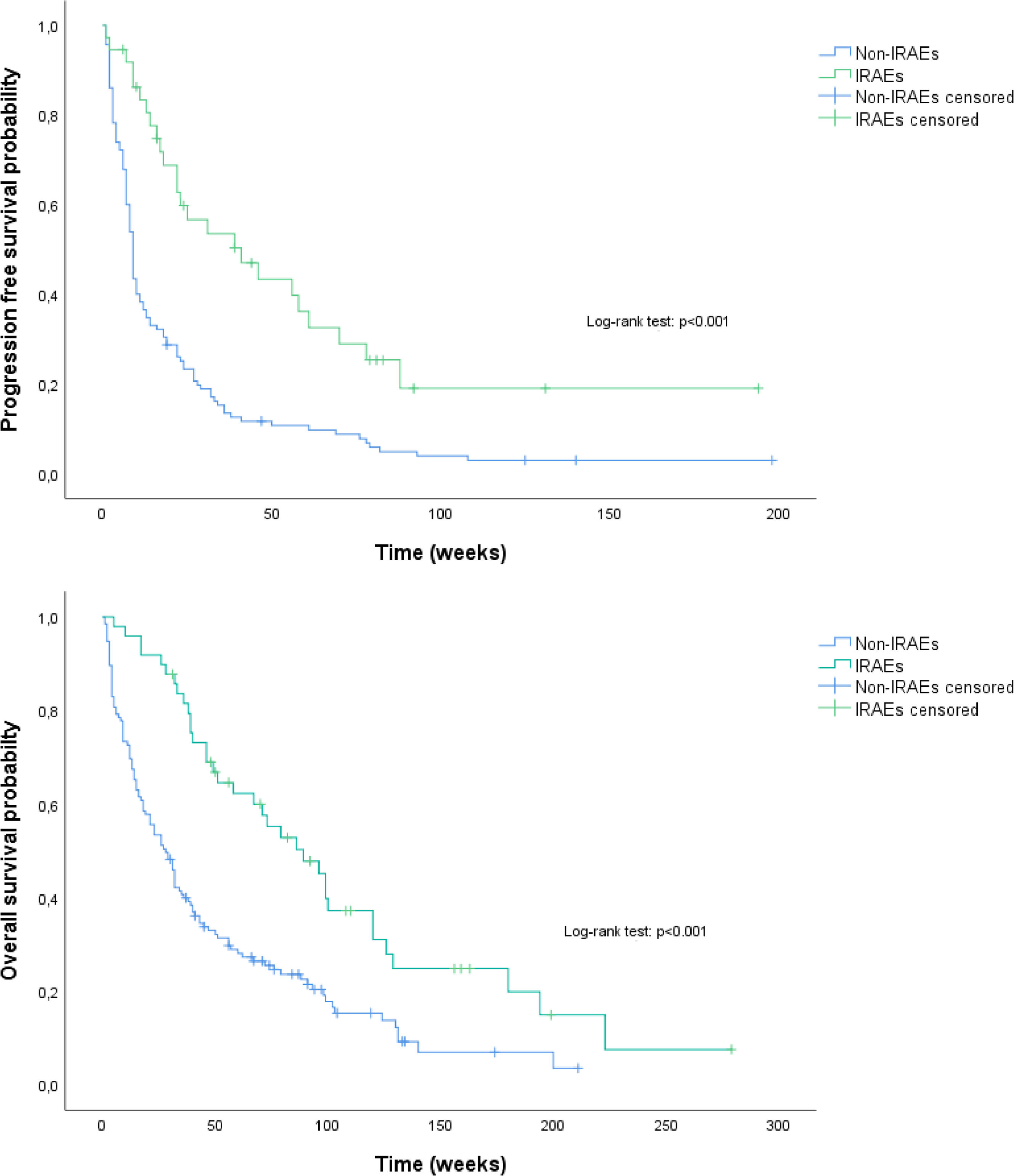
ResultsA total of 184 patients (77.7% men, mean age 66.9±9.5 years) treated with ICIs were analyzed. During follow-up, 49 (26.6%) patients developed IRAEs and 149 (81.0%) died. According to the multivariate logistic regression analysis, treatment with statins (OR:3.15; p = 0.007), previous systemic corticosteroid therapy (OR:3.99; p = 0.001), disease controlled as response to ICI (OR:5.93; p < 0.001) and higher hemoglobin values (OR:1.28; p = 0.040) were independent predictors for the development of IRAEs. Patients who developed IRAEs had significantly longer medians of PFS (41.0 vs 9.0 weeks, p < 0.001) and OS (89.0 vs 28.0 weeks; p < 0.001).
ConclusionsPatients treated with statins, pre-ICI systemic corticosteroids, higher baseline hemoglobin value and controlled disease as initial response to ICI had a higher risk of developing IRAEs. The development of IRAEs was associated with better outcomes.
Non-small cell lung cancer (NSCLC) accounts for the majority of all lung cancers,1,2 which in most cases present with metastatic disease at diagnosis1. Lung cancer is the leading cause of cancer deaths worldwide.1,2 Advances in understanding the biology of cancer and the mechanisms of oncogenesis have showed that NSCLC is a heterogeneous disease and multiple molecular and immunohistochemical targets have been recognized.3
Immune checkpoint inhibitors (ICIs) targeting either programmed cell death protein 1 (PD-1) or programmed cell death ligand 1 (PD-L1) have become part of the clinical approach for management of NSCLC.2,4,5 ICIs are indicated as first-line treatment in patients with advanced NSCLC harboring high PD-L1 expression (PD-L1≥50%) in absence of targetable drive oncogene molecular variant, or in combination with platinum-based chemotherapy in patients with advanced NSCLC and PD-L1<50%.4,5
ICIs are associated with a specific spectrum of immune-related adverse events (IRAEs),6-9 by increasing the activity of the immune system.7,8 IRAEs can involve any organ or system, most commonly skin, colon, lungs, endocrine glands and liver.6-9 Most IRAEs are self-limiting or resolve with systemic corticosteroids, but in some cases, these can be severe and life-threatening and require permanent discontinuation of treatment.6,7
Currently, the pathophysiological mechanisms of IRAEs are poorly understood6,7 and biomarkers and/or patient risk factors that predict toxicity are not recognized. However, with the increasing use of immunotherapy, it is important to identify patients at increased risk for IRAEs in order to better predict and manage them.
Therefore, in this retrospective cohort study, we aimed to identify predictors of IRAEs in patients with NSCLC treated with ICIs. In addition, we assessed associations between outcomes, including progression-free survival (PFS) and overall survival (OS), and the development of IRAEs.
Material and methodsStudy populationA retrospective analysis was performed of patients with NSCLC treated with antagonist monoclonal antibodies against programmed death-1 (PD-1) (Nivolumab and Pembrolizumab) and the programmed death ligand-1 (PD-L1) (Atezolizumab), between 2016 and 2020, in the Department of Pulmonology at the Centro Hospitalar Universitário de São João, Portugal. Patient data were entirely anonymized and authorized by the body Responsible for Access to Information (RAI) of Centro Hospitalar Universitário de São João. The registration protocol complies with the ethical guidelines of the Declaration of Helsinki and it was approved by the Ethics and Health Committee of Centro Hospitalar Universitário de São João on July 7, 2021.
Eligibility criteria were age ≥18 years, histological or cytological confirmation of NSCLC and treatment with ICI according to clinical practice. A minimum follow-up time of 4 weeks after starting the ICI was required for inclusion in the study. All patients with metastatic NSCLC and a targetable driver oncogene molecular variant (EGFR, ALK, or BRAF) had previously received targeted therapy for this oncogene prior to treatment with ICI.
Pembrolizumab and Atezolizumab were administered intravenously (iv) every 3 weeks at doses of 200 mg and 1200 mg, respectively. Nivolumab was administered iv at a dose of 240 mg every 2 weeks.
Data collectionDemographic and clinical characteristics of patients before starting ICIs were reviewed, including smoking history, comorbidities, systemic corticosteroid therapy in the 3 months prior to ICIs, Eastern Cooperative Oncology Group (ECOG) performance status (PS), tumor histology, molecular results and PD-L1 expression level (when available), disease stage, previous treatments, and baseline blood laboratory results. Baseline blood laboratory results were defined as the most recent (within 2 weeks) before the start of ICI, and were used to calculate NLR (absolute neutrophil count/absolute lymphocyte count) and PLR (platelet count/absolute lymphocyte count). The association between NLR≥5 and PLR≥180 values and outcomes were tested. These cutoff points were chosen according to literature references.10,11
Study assessmentsThe primary aim was to determine predictors of development of IRAEs. IRAEs were considered as adverse events related to an immune dysregulation that required specific monitoring or treatment. The Common Terminology Criteria for Adverse Events (CTACAE) of the National Institute (version 4.03) was used to assess patients’ adverse events. Based on IRAEs, patients were divided into two groups (IRAEs group and non-IRAEs group). Demographic and clinical characteristics and blood laboratory results were compared between groups.
ICIs were maintained until evidence of disease progression, death, severe or life-threatening IRAEs. Disease control rate (DCR) and pattern of disease progression during treatment with ICI were assessed. The Response Evaluation Criteria in Solid Tumors (RECIST) was applied for the evaluation of disease response. RECIST may underestimate the therapeutic benefit of ICIs, due to low accuracy in detecting pseudoprogression.4 However, in all cases, considering clinical and radiological findings, progression and DCR were admitted after consensus in a multidisciplinary lung cancer team meeting. DCR was defined as the proportion of participants with complete and partial response or disease stability for at least 6-8 weeks from onset of ICI.
The secondary objective was to assess the impact of developing IRAEs on PFS and OS. Progression-free survival (PFS) was calculated as the time from the start of ICI treatment to the date of radiographic or clinical progression or patient death. Overall survival (OS) was calculated from the time of initiation of the ICI until death from any cause or last follow-up.
Statistical analysisCategorical variables are presented as frequencies and percentages, and continuous variables as means and standard deviations (SD), or medians and interquartile ranges (IQR) for variables with skewed distribution. Normal distribution was tested using skewness and kurtosis. We used the chi-square test to compare categorical variables. Independent-samples t-test was used to evaluate differences in continuous variables with normal distribution and Mann-Whitney U tests were used to evaluate differences in continuous variables with skewed distribution. A univariate and multivariate binary logistic regression analysis were performed to determine predictors of development of IRAEs. PFS and OS curves were calculated using the Kaplan-Meier method, and the long-rank test was used to assess survival differences between groups. Univariate and multivariate Cox proportional hazards regression models were used to identify factors associated with PFS and OS. Factors which were statistically significant in the univariable/unadjusted analysis were incorporated into the multivariable/adjusted analysis. The p-value considered for statistical significance was 0.05. Data was stored and all statistical analyses were performed using the Statistical Package for Social Sciences (SPSS, IBM Corp, Chicago, IL, USA) software, version 26.0.
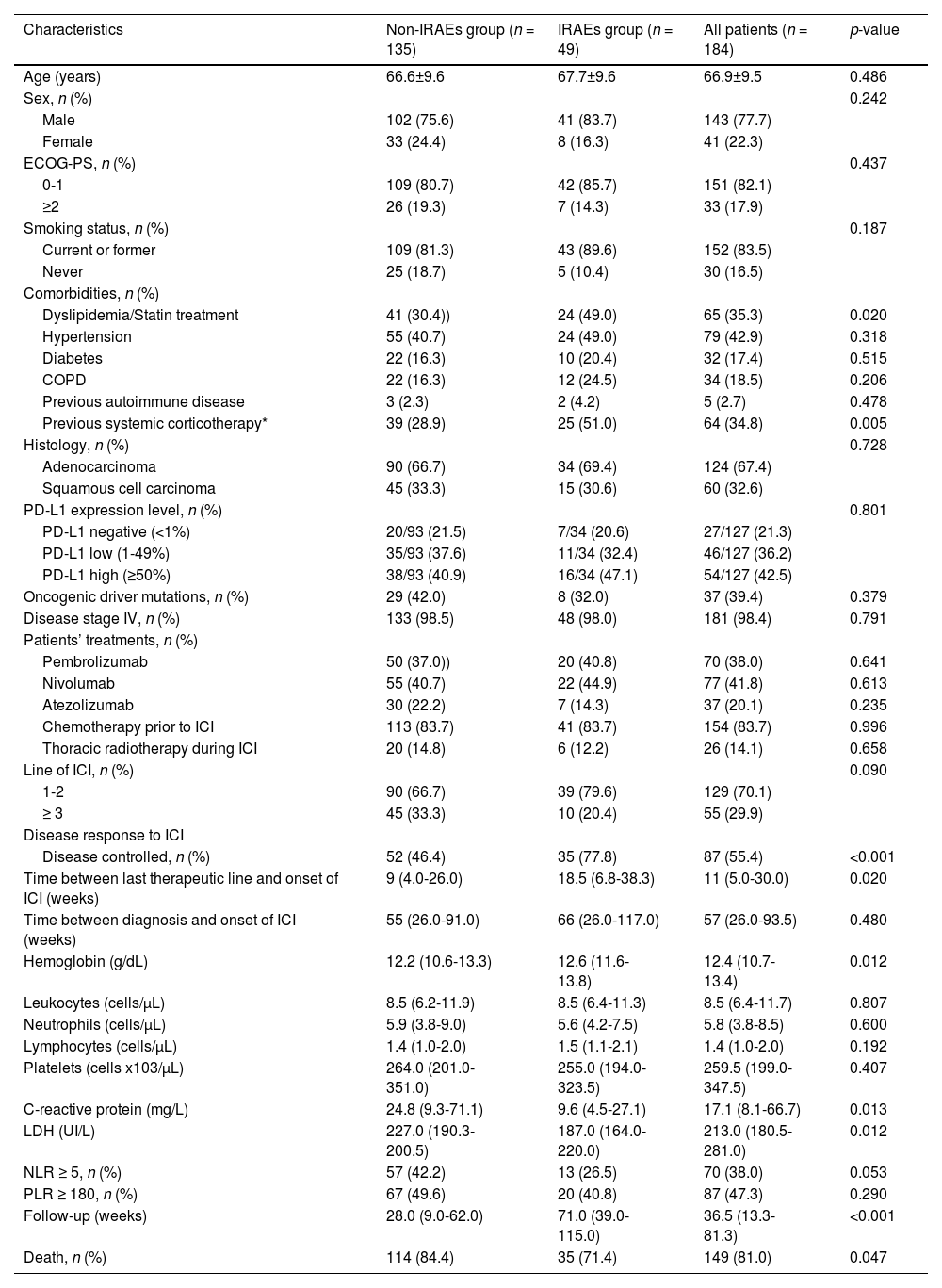
ResultsA total of 184 patients with NSCLC treated with ICIs were included. Patients had a mean age of 66.9±9.5 years and the majority were male (77.7%), current or former smokers (83.5%) and had an ECOG-PS of 0-1 (82.1%), non-squamous histology (67.4%), stage IV disease (98.4%) and PD-L1 expression level ≥1 (Table 1). Most patients were treated with ICIs in first- or second-line, 70 (38.0%) patients were treated with Pembrolizumab, 77 (41.8%) with Nivolumab and 37 (20.1%) with Atezolizumab (Table 1).
General characteristics of patients.
| Characteristics | Non-IRAEs group (n = 135) | IRAEs group (n = 49) | All patients (n = 184) | p-value |
|---|---|---|---|---|
| Age (years) | 66.6±9.6 | 67.7±9.6 | 66.9±9.5 | 0.486 |
| Sex, n (%) | 0.242 | |||
| Male | 102 (75.6) | 41 (83.7) | 143 (77.7) | |
| Female | 33 (24.4) | 8 (16.3) | 41 (22.3) | |
| ECOG-PS, n (%) | 0.437 | |||
| 0-1 | 109 (80.7) | 42 (85.7) | 151 (82.1) | |
| ≥2 | 26 (19.3) | 7 (14.3) | 33 (17.9) | |
| Smoking status, n (%) | 0.187 | |||
| Current or former | 109 (81.3) | 43 (89.6) | 152 (83.5) | |
| Never | 25 (18.7) | 5 (10.4) | 30 (16.5) | |
| Comorbidities, n (%) | ||||
| Dyslipidemia/Statin treatment | 41 (30.4)) | 24 (49.0) | 65 (35.3) | 0.020 |
| Hypertension | 55 (40.7) | 24 (49.0) | 79 (42.9) | 0.318 |
| Diabetes | 22 (16.3) | 10 (20.4) | 32 (17.4) | 0.515 |
| COPD | 22 (16.3) | 12 (24.5) | 34 (18.5) | 0.206 |
| Previous autoimmune disease | 3 (2.3) | 2 (4.2) | 5 (2.7) | 0.478 |
| Previous systemic corticotherapy* | 39 (28.9) | 25 (51.0) | 64 (34.8) | 0.005 |
| Histology, n (%) | 0.728 | |||
| Adenocarcinoma | 90 (66.7) | 34 (69.4) | 124 (67.4) | |
| Squamous cell carcinoma | 45 (33.3) | 15 (30.6) | 60 (32.6) | |
| PD-L1 expression level, n (%) | 0.801 | |||
| PD-L1 negative (<1%) | 20/93 (21.5) | 7/34 (20.6) | 27/127 (21.3) | |
| PD-L1 low (1-49%) | 35/93 (37.6) | 11/34 (32.4) | 46/127 (36.2) | |
| PD-L1 high (≥50%) | 38/93 (40.9) | 16/34 (47.1) | 54/127 (42.5) | |
| Oncogenic driver mutations, n (%) | 29 (42.0) | 8 (32.0) | 37 (39.4) | 0.379 |
| Disease stage IV, n (%) | 133 (98.5) | 48 (98.0) | 181 (98.4) | 0.791 |
| Patients’ treatments, n (%) | ||||
| Pembrolizumab | 50 (37.0)) | 20 (40.8) | 70 (38.0) | 0.641 |
| Nivolumab | 55 (40.7) | 22 (44.9) | 77 (41.8) | 0.613 |
| Atezolizumab | 30 (22.2) | 7 (14.3) | 37 (20.1) | 0.235 |
| Chemotherapy prior to ICI | 113 (83.7) | 41 (83.7) | 154 (83.7) | 0.996 |
| Thoracic radiotherapy during ICI | 20 (14.8) | 6 (12.2) | 26 (14.1) | 0.658 |
| Line of ICI, n (%) | 0.090 | |||
| 1-2 | 90 (66.7) | 39 (79.6) | 129 (70.1) | |
| ≥ 3 | 45 (33.3) | 10 (20.4) | 55 (29.9) | |
| Disease response to ICI | ||||
| Disease controlled, n (%) | 52 (46.4) | 35 (77.8) | 87 (55.4) | <0.001 |
| Time between last therapeutic line and onset of ICI (weeks) | 9 (4.0-26.0) | 18.5 (6.8-38.3) | 11 (5.0-30.0) | 0.020 |
| Time between diagnosis and onset of ICI (weeks) | 55 (26.0-91.0) | 66 (26.0-117.0) | 57 (26.0-93.5) | 0.480 |
| Hemoglobin (g/dL) | 12.2 (10.6-13.3) | 12.6 (11.6-13.8) | 12.4 (10.7-13.4) | 0.012 |
| Leukocytes (cells/µL) | 8.5 (6.2-11.9) | 8.5 (6.4-11.3) | 8.5 (6.4-11.7) | 0.807 |
| Neutrophils (cells/µL) | 5.9 (3.8-9.0) | 5.6 (4.2-7.5) | 5.8 (3.8-8.5) | 0.600 |
| Lymphocytes (cells/µL) | 1.4 (1.0-2.0) | 1.5 (1.1-2.1) | 1.4 (1.0-2.0) | 0.192 |
| Platelets (cells x103/µL) | 264.0 (201.0-351.0) | 255.0 (194.0-323.5) | 259.5 (199.0-347.5) | 0.407 |
| C-reactive protein (mg/L) | 24.8 (9.3-71.1) | 9.6 (4.5-27.1) | 17.1 (8.1-66.7) | 0.013 |
| LDH (UI/L) | 227.0 (190.3-200.5) | 187.0 (164.0-220.0) | 213.0 (180.5-281.0) | 0.012 |
| NLR ≥ 5, n (%) | 57 (42.2) | 13 (26.5) | 70 (38.0) | 0.053 |
| PLR ≥ 180, n (%) | 67 (49.6) | 20 (40.8) | 87 (47.3) | 0.290 |
| Follow-up (weeks) | 28.0 (9.0-62.0) | 71.0 (39.0-115.0) | 36.5 (13.3-81.3) | <0.001 |
| Death, n (%) | 114 (84.4) | 35 (71.4) | 149 (81.0) | 0.047 |
Data are presented as frequencies and percentages for categorical variables, as means and standard deviations (SD) for parametric continuous variables and as medians and interquartile ranges (IQR) for non-parametric continuous variables.
Definition of abbreviations: IRAEs: Immune-related adverse events; ECOG-PS: Eastern Cooperative Oncology Group performance status; COPD: Chronic obstructive pulmonary disease; PD-L1: Programmed death-ligand 1; ICI: Immune checkpoint inhibitors
The median follow-up time of patients after starting the ICI was 36.5 (13.3-81.3) weeks (Table 1). During this period, 49 (26.6%) patients developed IRAEs. The general characteristics of the patients according to the development of IRAEs are shown in table 1. There were no statistically significant differences regarding age, sex, ECOG-PS, PDL-1 expression level, ICI treatment line and burden of comorbidities between groups, except for dyslipidemia and systemic corticosteroid therapy within 3 months of onset of ICIs, which were more prevalent in the group that developed IRAEs. In addition, patients with IRAEs had a significantly higher disease control rate with ICI treatment compared to the group that did not develop IRAEs (p < 0.001).
Regarding baseline blood laboratory results (Table 1), patients with IRAEs had a significantly higher median of hemoglobin (p = 0.012) and eosinophils (p = 0.013) and lower median C-reactive protein (p = 0.013) and LDH (p = 0.012).
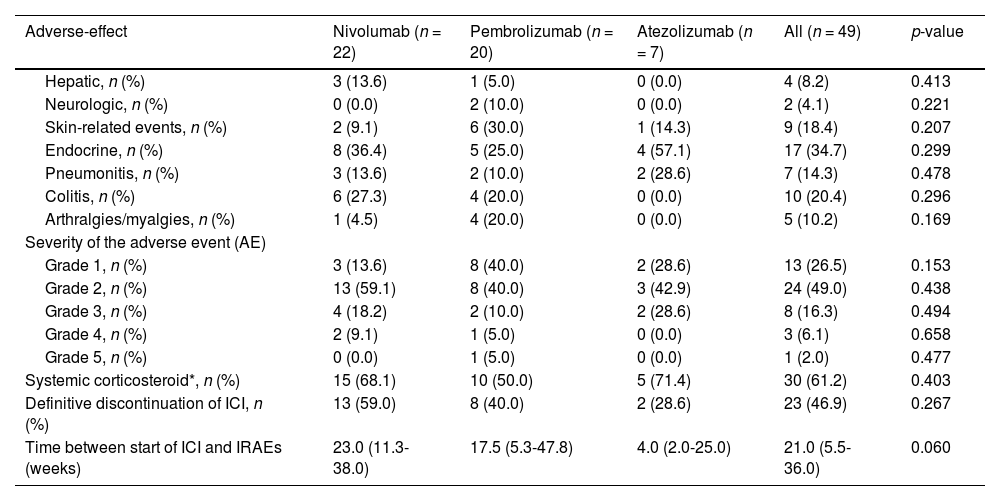
There was a similar rate of IRAEs in patients treated with Nivolumab (28.6%) and Pembrolizumab (28.6%), but a lower rate in patients treated with Atezolizumab (18.9%). The distribution of IRAEs by type of ICI is described in table 2.
Immune-related adverse events associated by type of ICI.
| Adverse-effect | Nivolumab (n = 22) | Pembrolizumab (n = 20) | Atezolizumab (n = 7) | All (n = 49) | p-value |
|---|---|---|---|---|---|
| Hepatic, n (%) | 3 (13.6) | 1 (5.0) | 0 (0.0) | 4 (8.2) | 0.413 |
| Neurologic, n (%) | 0 (0.0) | 2 (10.0) | 0 (0.0) | 2 (4.1) | 0.221 |
| Skin-related events, n (%) | 2 (9.1) | 6 (30.0) | 1 (14.3) | 9 (18.4) | 0.207 |
| Endocrine, n (%) | 8 (36.4) | 5 (25.0) | 4 (57.1) | 17 (34.7) | 0.299 |
| Pneumonitis, n (%) | 3 (13.6) | 2 (10.0) | 2 (28.6) | 7 (14.3) | 0.478 |
| Colitis, n (%) | 6 (27.3) | 4 (20.0) | 0 (0.0) | 10 (20.4) | 0.296 |
| Arthralgies/myalgies, n (%) | 1 (4.5) | 4 (20.0) | 0 (0.0) | 5 (10.2) | 0.169 |
| Severity of the adverse event (AE) | |||||
| Grade 1, n (%) | 3 (13.6) | 8 (40.0) | 2 (28.6) | 13 (26.5) | 0.153 |
| Grade 2, n (%) | 13 (59.1) | 8 (40.0) | 3 (42.9) | 24 (49.0) | 0.438 |
| Grade 3, n (%) | 4 (18.2) | 2 (10.0) | 2 (28.6) | 8 (16.3) | 0.494 |
| Grade 4, n (%) | 2 (9.1) | 1 (5.0) | 0 (0.0) | 3 (6.1) | 0.658 |
| Grade 5, n (%) | 0 (0.0) | 1 (5.0) | 0 (0.0) | 1 (2.0) | 0.477 |
| Systemic corticosteroid*, n (%) | 15 (68.1) | 10 (50.0) | 5 (71.4) | 30 (61.2) | 0.403 |
| Definitive discontinuation of ICI, n (%) | 13 (59.0) | 8 (40.0) | 2 (28.6) | 23 (46.9) | 0.267 |
| Time between start of ICI and IRAEs (weeks) | 23.0 (11.3-38.0) | 17.5 (5.3-47.8) | 4.0 (2.0-25.0) | 21.0 (5.5-36.0) | 0.060 |
Data are presented as frequencies and percentages for categorical variables and as medians and interquartile ranges (IQR) for non-parametric continuous variables.
Definition of abbreviations: ICI: Immune checkpoint inhibitors; IRAEs: Immune-related adverse events.
The majority (75.5%) of IRAEs were mild or moderate, but there were life-threatening IRAEs in 2 patients treated with Nivolumab and in 1 patient treated with Pembrolizumab and one fatal IRAE in 1 patient treated with Pembrolizumab. Thirty patients required systemic corticosteroid therapy and 23 definitive discontinuations of ICI due to IRAEs. Patients treated with Atezolizumab tended to have earlier IRAEs (p = 0.060), mostly endocrine-IRAEs.
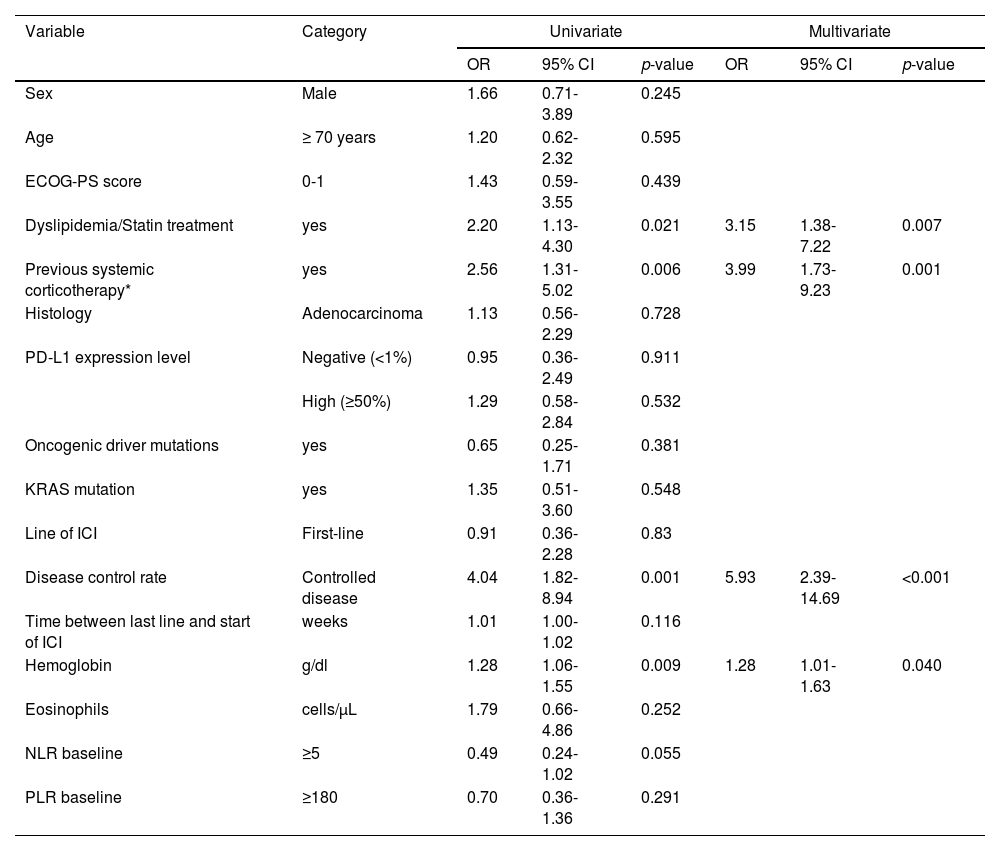
According to the univariate analysis (Table 3), patients with dyslipidemia, systemic corticoid therapy prior to ICI, controlled disease and higher hemoglobin values had a significantly higher risk of developing IRAEs. These associations were confirmed by multivariate logistic regression analysis, showing that dyslipidemia (OR: 3.15; 95% CI: 1.38-7.22, p = 0.007), previous systemic corticosteroid therapy (OR: 3.99; 95% CI: 1.73-9.23, p = 0.001), disease controlled as response to ICI (OR: 5.93; 95% CI: 2.39-14.69, p <0.001) and higher hemoglobin values (OR: 1.28; 95% CI: 1.01-1.63, p = 0.040) were independent predictors for the development of IRAEs (Table 3). Age, sex, ECOG-PS, histology, PD-L1 expression level, oncogenic driver mutations (including KRAS mutation), line of ICI and other analytical parameters were not associated with increased risk of IRAEs. The severity of IRAEs between dyslipidemic and non-dyslipidemic patients was evaluated, a statistically significant association was not found (p=0.456) (Fig. 2 in supplement).
Univariate and multivariate analyses to determine risk factors for IRAEs.
| Variable | Category | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | ||
| Sex | Male | 1.66 | 0.71-3.89 | 0.245 | |||
| Age | ≥ 70 years | 1.20 | 0.62-2.32 | 0.595 | |||
| ECOG-PS score | 0-1 | 1.43 | 0.59-3.55 | 0.439 | |||
| Dyslipidemia/Statin treatment | yes | 2.20 | 1.13-4.30 | 0.021 | 3.15 | 1.38-7.22 | 0.007 |
| Previous systemic corticotherapy* | yes | 2.56 | 1.31-5.02 | 0.006 | 3.99 | 1.73-9.23 | 0.001 |
| Histology | Adenocarcinoma | 1.13 | 0.56-2.29 | 0.728 | |||
| PD-L1 expression level | Negative (<1%) | 0.95 | 0.36-2.49 | 0.911 | |||
| High (≥50%) | 1.29 | 0.58-2.84 | 0.532 | ||||
| Oncogenic driver mutations | yes | 0.65 | 0.25-1.71 | 0.381 | |||
| KRAS mutation | yes | 1.35 | 0.51-3.60 | 0.548 | |||
| Line of ICI | First-line | 0.91 | 0.36-2.28 | 0.83 | |||
| Disease control rate | Controlled disease | 4.04 | 1.82-8.94 | 0.001 | 5.93 | 2.39-14.69 | <0.001 |
| Time between last line and start of ICI | weeks | 1.01 | 1.00-1.02 | 0.116 | |||
| Hemoglobin | g/dl | 1.28 | 1.06-1.55 | 0.009 | 1.28 | 1.01-1.63 | 0.040 |
| Eosinophils | cells/µL | 1.79 | 0.66-4.86 | 0.252 | |||
| NLR baseline | ≥5 | 0.49 | 0.24-1.02 | 0.055 | |||
| PLR baseline | ≥180 | 0.70 | 0.36-1.36 | 0.291 | |||
Definition of abbreviations: IRAEs: Immune-related adverse events; ECOG-PS: Eastern Cooperative Oncology Group performance status; PD-L1: Programmed death-ligand 1; ICI: Immune checkpoint inhibitor; NLR: Neutrophil-to-lymphocyte ratio; PLR: Platelet-to-lymphocyte ratio; OR: odds ratio; CI: confidence interval.
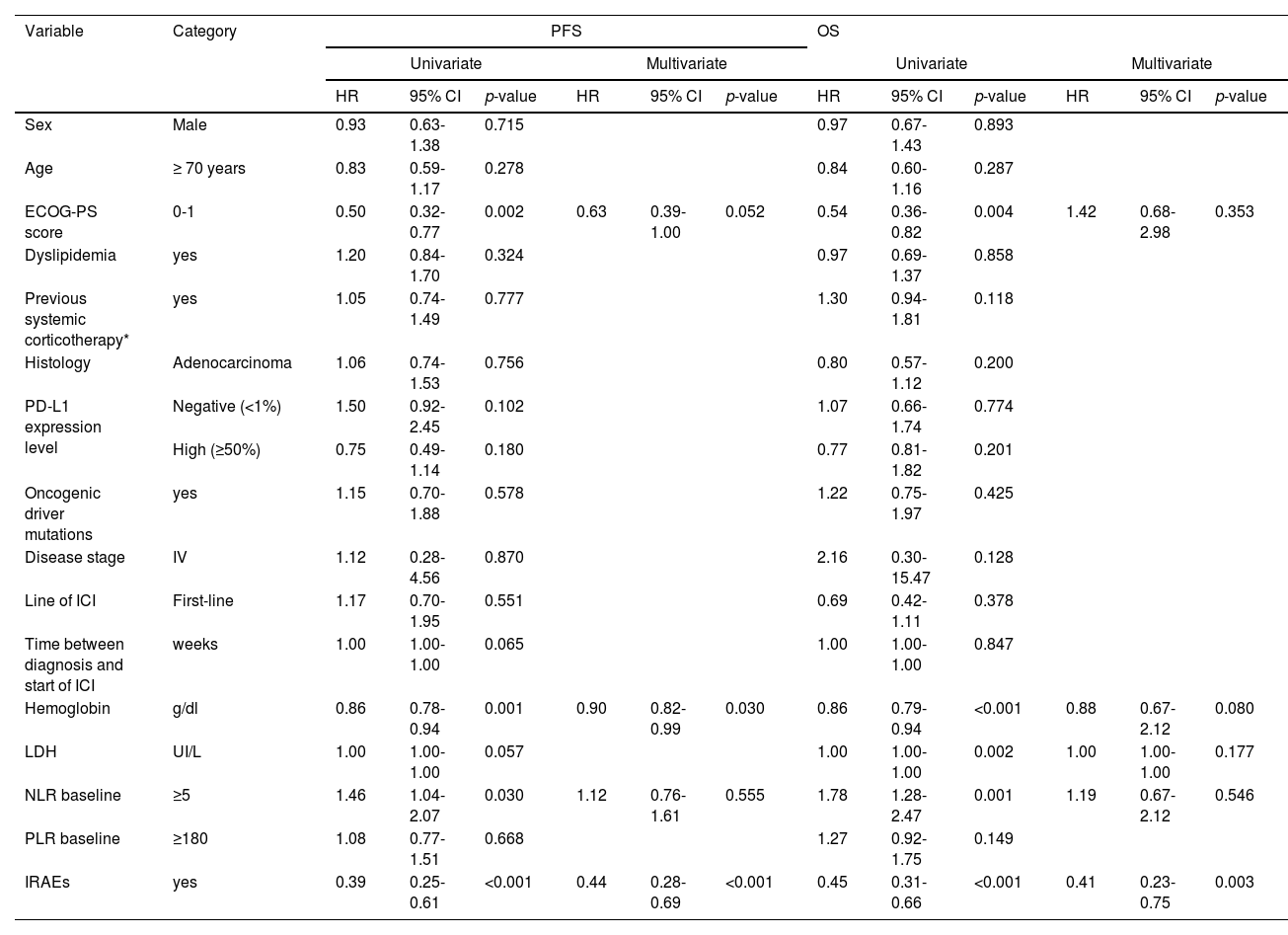
During the follow-up period, 149 (81.0%) patients died. Patients with IRAEs had a significantly longer median PFS compared to patients without IRAEs (41.0 weeks vs 9.0 weeks, p < 0.001) (Fig. 1.A). The univariate analysis identified ECOG-PS score of 0-1 (p = 0.002), a higher hemoglobin value (p = 0.001) and the development of IRAEs (p < 0.001) as factors associated with improved PFS; a baseline NLR≥5 (p = 0.030) was associated with worse PFS (Table 4). A higher hemoglobin value (HR: 0.90; 95% CI: 0.82-0.99, p = 0.030) and the development of IRAEs (HR: 0.44; 95% CI: 0.28-0.69, p < 0.001) persisted significantly associated with improved PFS in the multivariate analysis.
Univariate and multivariate analyses of PFS and OS.
Definition of abbreviations: PFS: Progression-free survival; OS: Overall survival; IRAEs: Immune-related adverse events; ECOG-PS: Eastern Cooperative Oncology Group performance status; PD-L1: Programmed death-ligand 1; ICI: Immune checkpoint inhibitors; NLR: Neutrophil-to-lymphocyte ratio; PLR: Platelet-to-lymphocyte ratio; HR: Hazard ratio; CI: confidence interval.
Median OS was also significantly higher in patients who developed IRAEs (89.0 vs 28.0 weeks, p < 0.001) (Fig. 1.B). In the univariate analysis, ECOG-PS score 0–1 (p = 0.004), hemoglobin value (p < 0.001), LDH value (p = 0.002), NLR ≥5 (p = 0.001), and the development of IRAEs (p < 0.001) was significantly associated with overall survival. In the adjusted analysis, only the development of IRAEs was significantly associated with better OS (HR: 0.41; 95% CI: 0.23-0.75, p = 0.003) (Table 4).
DiscussionICIs are currently one of the mainstays of NSCLC treatment. However, not infrequently, patients treated with ICIs develop IRAEs.6-9
In this study, our purpose was to identify predictors of IRAEs in patients with NSCLC treated with ICIs. Similar to other studies,13,14 in our analysis more than a quarter of patients developed IRAEs (grade≥2 in 73.5% of cases). In most cases with IRAEs, systemic corticosteroid therapy was required and almost half of them had to permanently discontinue ICI. Lower rates of definitive discontinuation of ICIs due to IRAEs have been reported,6,15,16 but the majority of studies assessing IRAEs had a shorter follow-up period than ours.
In addition, we showed that there was a similar rate of IRAEs in patients treated with Nivolumab and Pembrolizumab and a lower rate in patients treated with Atezolizumab. However, a smaller number of patients treated with Atezolizumab were analyzed, so the association between the type of ICI and increased risk of IRAEs cannot be established based on our results.
Taking into account our purpose, we found that dyslipidemia, pre-ICI systemic corticosteroids, disease controlled as response to ICI and a higher baseline hemoglobin value were independent predictors of the development of IRAEs. Regarding these results, we emphasize that all dyslipidemic patients were treated with statins. Statins have been associated with an immunomodulatory effect by preventing protein prenylation, increasing antigen presentation, T cell activation and cytolytic response.17 Some studies have even suggested that statins may act synergistically with ICIs,18 potentiate their effects18 and be associated with better outcomes in lung cancer patients.19,20 So, the increased risk of IRAEs in patients with dyslipidemia that we found may be mainly related to treatment with statins. Despite this, we did not find a statistically significant association between dyslipidemia/statin use and the severity of IRAEs, and dyslipidemia/statin use did not have a relevant impact on PFS or OS. Thus, the association between the use of statins and the risk of IRAEs, as well as the impact on outcomes in patients treated with ICIs, should be further explored.
Concerning corticosteroid therapy, Arbor et al.21 previously reported that the use of ≥10 mg prednisone equivalent at the time of initiation of ICI is associated with a worse outcome in patients with NSCLC. Here, about a third of patients were treated with systemic corticosteroids prior to initiation of ICI, in most cases to control symptoms directly or indirectly related to the disease. Despite this, at the start of ICI, all patients had stopped or received an equivalent dose of prednisone <10 mg daily. So, we assessed the impact of systemic corticosteroid treatment before starting ICI on the development of IRAEs and outcomes, and we found that pre-ICI corticosteroid treatment was associated with a higher risk of IRAEs, but not with worse PFS or OS. In our opinion, this association may identify a subgroup of patients with T-cells that are more responsive in proliferation and differentiation to the modulating effect of ICIs and corticosteroids. Although corticosteroid treatment at the start of ICI may be associated with a negative impact on clinical outcomes, based on our results, there is no association if corticosteroid use is prior to ICI. In fact, studies that showed an association between the use of corticosteroids (at the beginning of ICI) and worse outcomes do not exclude the possibility that these patients had more comorbidities and a poorer baseline prognosis.21,22
Furthermore, in our analysis, a higher baseline hemoglobin value was associated with the development of IRAEs and better outcomes. Other studies had reported the hemoglobin value as a strong predictor of response (independent of performance status) to treatment with ICIs in NSCLC patients.23 Anemia is a common abnormality in patients diagnosed with advanced cancer,24 and there is evidence that anemia and hypoxemia may be implicated in tumor growth, anti-apoptosis and angiogenesis mechanisms, and reduced effects of therapies.25 Thus, it is plausible that higher hemoglobin values may be associated with a greater effect of ICI's, better outcomes, but also with more IRAEs. Therefore, correction of anemia before starting ICIs may have clinical benefits.
In NSCLC, inflammatory biomarkers have shown to correlate with a poor prognosis and a low therapeutic response to conventional treatment.10,12 Some studies have even shown an association between baseline NLR and PLR and the risk of IRAEs and prognostic outcomes.10,12 Using published cutoff points of NLR11 and PLR,12 we found an association between baseline NLR≥5 and worse PFS and OS in univariate analysis, but this association was not confirmed in multivariate analysis. In addition, the association between baseline NLR or PLR and the risk of IRAEs was not found. Nevertheless, these results may be due to the considerable number of patients treated with systemic corticosteroids pre-ICI, by modulating peripheral blood immune cells profile.
Moreover, we showed that patients with IRAEs had a significantly higher disease control rate in response to ICI and significantly longer median PFS and OS compared to patients without IRAEs. The association between the development of IRAEs and better PFS and OS was supported by univariate and multivariate analyses. Several studies have already shown an association between IRAEs and better outcomes in patients with metastatic melanoma26-28 and NSCLC.14,29-35 Regarding this, Biagio Ricciuti et al.35 showed that the development of IRAEs was even a strong predictor of PFS and OS outcomes in NSCLC patients treated with Nivolumab.
As mentioned above, although the mechanisms of IRAEs are not fully understood, this is certainly related to the hyperstimulation of the immune system by ICIs. Therefore, patients with a greater immune system response to ICI will have better antitumor responsiveness and a higher risk of developing adverse effects.
There are some limitations to our study. This is a unicentric retrospective study so information bias cannot be excluded. IRAEs were considered when recorded by patients' physicians and mild IRAEs may not have been valued and not recorded. The small number of cases in our study may have affected some results and the interpretation of predictors and outcomes. The individual impact of each therapeutic strategy on outcomes has not been assessed.
Despite this, we present some relevant results, highlighting the value of hemoglobin and the use of statins rather than inflammatory biomarkers, which have been more traditionally reported as predictors of IRAEs and outcomes in patients treated with ICIs.
ConclusionsIn conclusion, the most important results of our study were that patients treated with statins, pre-ICI systemic corticosteroids, higher baseline hemoglobin values, and controlled disease as an initial response to ICI had an increased risk of developing IRAEs. Furthermore, patients who developed IRAEs had better outcomes compared to patients who did not. Further studies with a larger number of patients are needed to validate and complement these results, as well as to better clarify the mechanisms of how ICIs act, in order to achieve the best efficacy and minimal toxicity with this therapy.
CRediT authorship contribution statementM. Serino: Conceptualization, Methodology, Investigation, Data curation, Resources, Software, Formal analysis, Writing – original draft, Visualization, Writing – review & editing. C. Freitas: Conceptualization, Methodology, Investigation, Data curation, Resources, Formal analysis, Writing – original draft, Visualization, Writing – review & editing. M. Martins: Conceptualization, Investigation, Resources, Visualization. P. Ferreira: Conceptualization, Investigation, Resources, Visualization. C. Cardoso: Conceptualization, Investigation, Resources, Writing – original draft, Visualization. F. Veiga: Investigation, Resources. V. Santos: Conceptualization, Methodology, Writing – original draft, Visualization. D. Araújo: Conceptualization, Methodology, Writing – original draft, Visualization. H. Novais-Bastos: Conceptualization, Methodology, Investigation, Data curation, Resources, Formal analysis, Writing – original draft, Visualization, Writing – review & editing. A. Magalhães: Conceptualization, Methodology, Writing – original draft, Visualization. H. Queiroga: Conceptualization, Methodology, Writing – original draft, Visualization. G. Fernandes: Conceptualization, Methodology, Investigation, Data curation, Resources, Formal analysis, Writing – original draft, Visualization, Writing – review & editing. V. Hespanhol: Conceptualization, Methodology, Investigation, Data curation, Resources, Formal analysis, Writing – original draft, Visualization, Writing – review & editing.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.