Despite its importance, there are some barriers to patient compliance in preventive therapy (PT) of tuberculosis (TB). The purpose of this study was to evaluate the compliance to appointments, PT and follow-up in a pediatric population after TB exposure, followed in a single TB outpatient center, and the subsequent identification of compliance determinants.
MethodsRetrospective analysis of all pediatric patients who underwent PT in Gaia TB outpatient center from January 2015 to June 2016. Patients were divided into two groups: compliant and non-compliant, according to adherence to screening, visits and medication. The data collection was based on review of medical records.
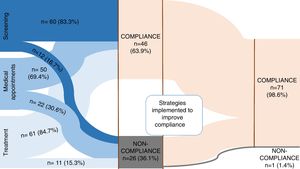
ResultsA total of 72 patients were enrolled, 33 (45.8%) on chemoprophylaxis and 39 (54.2%) on latent tuberculosis infection (LTBI) treatment. The majority of patients were compliant (63.9%, n=46). Non-compliance was found in 36.1% (n=26): in 12 patients to contact screening, in 11 patients to PT and 22 patients did not attend medical appointments in the first place. In 10 patients, non-compliance was related to social problems/family dysfunction (low socioeconomic status and parent's unemployment). After putting in place several strategies, such as telephone contact, activating social services and direct observation of therapy, a compliance of 98.6% was achieved. Isoniazid was the main drug used (91.7%), during 9 months for LBTI.
ConclusionPT compliance in TB can be challenging, probably related to the lack of risk perception and caregiver's reluctance to undergo a prolonged treatment to an asymptomatic condition. We conclude that implementing interventions can considerably improve treatment compliance and reduce the risk of future tuberculosis development. We emphasize the success in compliance to a 9 month regimen of isoniazid in the vast majority of patients with LTBI.
Tuberculosis (TB) remains an important infectious disease in pediatric age group.1–3 Although a low incidence threshold for tuberculosis was achieved in 2015 in Portugal (incidence of tuberculosis<20/100,000 habitants),4 children remain at higher risk for developing tuberculosis.
After Mycobacterium tuberculosis (Mt) infection, children, especially those under 6 years old, have a higher probability of developing the disease, usually in the first two years following infection.1,5 TB contact screening and implementation of preventive therapy (PT) remain as important measures to reduce the risk of progression to TB.5 TB contact screening is carried out in TB outpatient centers after TB exposure and in candidates to immunosuppressive therapies.6
PT is indicated when latent tuberculosis infection (LTBI) is diagnosed and as chemoprophylaxis (CP) in children under 6 years old after TB exposure.
LTBI diagnose is based on a positive immunological test, and PT is continued with isoniazid for 6–9 months. Alternative regimens, such as 4 months rifampicin, may be an option in cases of resistance to isoniazid, or adverse events. Efficacy is described in the literature as 90% with 9-months of isoniazid, 69% with 6-months of isoniazid and 59% with 3–4 months of rifampicin.6,7 Most recently, another regimen has emerged, with twelve doses of weekly rifapentine plus and isoniazid for 3 months. This last regimen has shown to be equivalent to isoniazid in children aged 2 years and older, but with higher compliance.6,8
There has been some controversy about the best PT,9,10 with some authors defending shorter anti-TB drugs regimens in order to improve compliance.8,11 Compliance to a long-term daily treatment is crucial to TB control. The primary objective of this study was to determine the compliance rate to TB PT in a cohort of children and adolescents receiving TB drugs as primary chemoprophylaxis, or as LTBI treatment, identifying failure compliance determinants. The evaluation of implementation of strategies to improve PT compliance was a secondary objective of this study.
MethodsWe carried out a retrospective cohort study, based on medical records review, on the compliance to PT (CP or LTBI treatment) and to scheduled appointments in pediatric patients (<18 years of age) followed-up from January 2015 to June 2016 at Gaia outpatient TB center, a TB reference center at the north region of Portugal. This center is a community referral center for tuberculosis in the city of Gaia, Portugal, where all children/adolescents of this region who have been exposed to someone with tuberculosis or with suspected tuberculosis disease are screened, investigated and treated, including patients with Mt sensitive to antitubercular drugs and multidrug-resistant cases. Chemoprophylaxis was prescribed after TB contact with a smear-positive patient for children younger than 6 years, after exclusion of active tuberculosis and discontinued 8–12 weeks later, after a second negative screening. The drug of choice was isoniazid, unless index case was resistant to isoniazid, in which case rifampicin was chosen. LTBI diagnosis was made in case of positive IGRA/TST in children younger than 6 years of age or both positive TST and IGRA in children ≥6 years old, asymptomatic and with normal chest X-ray. LTBI treatment consisted of a 9-month regimen of isoniazid monotherapy or, in case of resistance to isoniazid of index case or intolerance/side effects to isoniazid, 4-months regimen of rifampicin. Medication was given to parents/caregivers once a week, free of charge, daily dosing was indicated and administered by parents at home. All patients on PT were seen monthly until the treatment was complete both to improve compliance and to check for symptoms or adverse effects. Demographic characteristics, clinical findings at admission, side effects to the regimens, workup and strategies to improve compliance, were collected from patient medical record. Patients were divided into two groups: (a) compliant – those patients who attended all the scheduled appointments/screening procedures and completed the proposed PT; (b) non-compliant – considered to be failure on medication regimen or presence of other factors that raised the suspicion of non-compliance (missing to one or more medical appointment or screening). Reasons for non-compliance were classified from the perspective of the patient's medical doctor through review of clinical records. Social problems/family dysfunction were defined by the presence of any of the following criteria: (1) families in which conflict and child neglect occurred and was noticed during medical appointments; (2) families shown to be at risk by the protection commission for children and adolescents; (3) alcohol or drug abuse of any of the parents; (4) low socioeconomic status and parental unemployment. All data analyses were performed using the SPSS, version 24.0. Statistical significance was determined at the level of p<0.05. Confidence intervals were set at 95%. Categorical variables are described as frequencies and percentages, and continuous variables as means and standard deviation or medians and interquartile ranges, respectively. Differences between compliant and non-compliant were tested using χ2 test or Fisher exact test for categorical variables and Student's t-test or Mann–Whitney test for independent samples, as appropriate.
ResultsA total of 72 patients were enrolled, 33 (45.8%) on CP and 39 (54.2%) on LTBI. The overall results are synthesized in Fig. 1.
Sociodemographic data are described in Table 1. The median age was 5.5 years, and it was significantly lower in the CP group comparing with LTBI group (2.9 vs. 7.7 years, respectively; p<0.001). Globally there was a male predominance. Patients were referred to our center mostly by public health services, especially after exposure to tuberculosis (n=63). The index case was intrafamilial in the majority of patients (79.2%), with a predominance of grandparents (n=20); with a daily contact (n=35). Mt of the index case was susceptible to all drugs in 90% of cases. There were 4 patients referred to screening for immune mediated inflammatory diseases candidates for biologic therapy or other immunosuppressive agents. Patients were vaccinated with BCG-vaccine (100%, n=68; 4 missing values), according to the universal BCG vaccination standard in practice at that time. At the time of the first medical consultation, 17 patients (23.6%) had symptoms (cough and/or fever). Isonazid was started in 67 patients (93.1%) and rifampicin in 5 patients (6.9%, for isoniazid-resistant Mt of the index case). In case of CP, treatment was continued for a mean of 9.7±3.1 weeks and till a second screening ruled out LTBI. The second screening was preformed 9.7 weeks after the first one and included TST and IGRA. Complete blood count and liver function tests were performed in 33.4% of patients (n=24) after the initiation of treatment, with normal results.
Sociodemographic and clinical characteristics.
| Variable | Total groupn=72 | Compliancen=46 | Non-compliancen=26 | p-Value |
|---|---|---|---|---|
| Age, years, mean±SD | 5.5±4.2 | 4.8±3.8 | 6.9±4.7 | 0.046 |
| Male, No. (%) | 40 (55.6) | 29 (63.0) | 11 (42.3) | 0.089 |
| Index case | ||||
| Mother, No. (%) | 12 (16.7) | 7 (15.2) | 5 (19.2) | 0.746 |
| Father, No. (%) | 9 (12.5) | 7 (15.2) | 2 (7.7) | 0.473 |
| Brother/sister, No. (%) | 2 (2.8) | 0 (0) | 2 (7.7) | 0.127 |
| Grandparents, No. (%) | 20 (27.8) | 14 (30.4) | 6 (23.1) | 0.503 |
| Uncle/aunt, No. (%) | 14 (19.4) | 8 (17.4) | 6 (23.1) | 0.558 |
| Other, No. (%) | 10 (13.9) | 7 (15.2) | 3 (11.5) | 0.739 |
| No index case identified, No. (%) | 5 (6.9) | 3 (6.5) | 2 (7.7) | 0.999 |
| Contact with index case* | ||||
| Daily, No. (%) | 35 (63.6) | 22 (61.1) | 13 (68.4) | 0.592 |
| Weekly, No. (%) | 11 (20.0) | 8 (22.2) | 3 (15.8) | 0.730 |
| Sporadic, No. (%) | 9 (16.4) | 6 (16.7) | 3 (15.8) | 0.999 |
| Tuberculosis of the index case† | ||||
| Pulmonar, No. (%) | 59 (92.2) | 36 (87.8) | 23 (100) | 0.150 |
| Pleuropulmonar, No. (%) | 3 (4.7) | 3 (7.3) | 0 (0) | 0.547 |
| Miliar, No. (%) | 2 (3.1) | 2 (4.9) | 0 (0) | 0.532 |
| M. tuberculosis of the index case‡ | ||||
| Susceptible to all drugs, No. (%) | 54 (90.0) | 33 (86.8) | 21 (95.5) | 0.400 |
| Resistant to isoniazid, No. (%) | 5 (8.3) | 4 (10.5) | 1 (4.5) | 0.643 |
| Multidrug resistant, No. (%) | 1 (1.7) | 1 (2.6) | 0 (0) | 0.999 |
| Drug of choice, No. (%) | ||||
| Isoniazid | 67 (93.1) | 42 (91.3) | 25 (96.2) | 0.647 |
| Rifampicin | 5 (6.9) | 4 (8.7) | 1 (3.8) | 0.647 |
| Origin of the patient, No. (%) | ||||
| Public health | 61 (84.7) | 39 (84.8) | 22 (84.6) | 0.999 |
| Family doctor | 4 (5.6) | 3 (6.5) | 1 (3.8) | 0.999 |
| Emergency department | 1 (1.4) | 1 (2.2) | 0 (0) | 0.999 |
| Oncology department | 1 (1.4) | 0 (0) | 1 (3.8) | 0.361 |
| Pediatrician appointment | 4 (5.6) | 3 (6.5) | 1 (3.8) | 0.999 |
| Inpatient department | 1 (1.4) | 0 (0) | 1 (3.8) | 0.361 |
| Reason for referral, No. (%) | ||||
| Exposure to TB | 63 (87.5) | 39 (84.8) | 24 (92.3) | 0.473 |
| Suspicion of active TB | 5 (6.9) | 4 (8.7) | 1 (3.8) | 0.647 |
| Candidate to immunosuppressive treatment | 4 (5.6) | 3 (6.5) | 1 (3.8) | 0.999 |
| Type of preventive therapy, No. (%) | ||||
| CP | 33 (45.8) | 25 (75.8) | 8 (24.2) | 0.054 |
| LTBI treatment | 39 (54.2) | 21 (53.8) | 18 (46.2) | 0.054 |
| Family disfunction, No. (%) | 10 (13.9) | 0 (0) | 10 (38.5) | § |
| Medication problems, No. (%) | 10 (13.9) | 0 (0) | 10 (38.5) | § |
SD: standard deviation; CP: chemoprophylaxis; LTBI: latent tuberculosis infection.
There was compliance to screening, visits and treatment in 63.9% (n=46) and non-compliance in 36.1% (n=26; Fig 1). A stratified analysis of the results according to the type of treatment (CP vs. LTBI) revealed a compliance of 75.8% (n=25) in CP group and 53.8% (n=21) in LTBI, p=0.054. Patient age was significantly higher in non-compliant group (6.9±4.7 years-old vs. to 4.8±3.8 in compliance group, p=0.046). Social problems/family dysfunction were present in 38.5% (n=10) patients, all non-compliant ones.
Missing appointments were registered in 30.6% (n=22) and were related with age ≥6 years old (46.2% vs. 21.9% in children <6 years old; p=0.031). Of those who missed appointments, 36.4% (n=8) failed to complete the treatment. There was an association between missing appointments and failure in treatment (p=0.002). A group of 14 patients maintained treatment despite missing medical appointments (19.4%) and this group was significantly older (mean age 8.9±4.0 vs. 4.8±3.8 years old; p=0.003) and mostly on LTBI treatment (n=12; 85.7%). Patients in CP had a median of 4 (IQR 3–6) medical appointments and LTBI patients a median of 7 (IQR 4:8).
The reasons found for non-compliance are described in Fig. 2, and included social problems/family dysfunction and medication problems, which consisted of symptoms related with medication such as nausea, vomiting or other gastrointestinal symptoms, side effects and intolerance to treatment. Non-compliance to contact screening was found in 12 patients and in 11 patients (15.3%) to PT. In 10 patients, non-compliance was related to family dysfunction/social problems. Medication side effects were seen in 3 patients (4.2%), with one patient needing to change isoniazid to rifampicin (9.1%), with subsequent compliance to treatment. Oral intolerance to medication was seen in 1 patient (9.1%). For 2 patients there was no explanation found to non-compliance to treatment. Follow-up of patients was monthly until treatment was complete.
When non-compliance of any kind was noticed, some strategies were implemented (Fig 2): all parents/caregivers were contacted by phone and encouraged to return to the appointments and take the medication, rescheduling a new appointment (n=26; 100%); social service was activated in order to help the return of these families to the appointments (n=2; 7.7%); directly observed treatment was implemented (n=1; 3.8%); change in medication (3.8%) and shortening of the time of prescription ensuring regular and closer monitoring of drug supply (7.7%). With the implementation of these strategies, a final compliance rate of 98.6% was achieved (n=71). There was 1 case of loss of follow-up. Isoniazid was the main drug used (n=66; 91.7%), in 31 cases of CP with a median duration of 9 (IQR 8:12) weeks and in 35 cases of LTBI for 9 months, with a compliance of 97.1% to 9-month regimen with isoniazid. Rifampicin was used for four months in 8.3% (n=6), one for side effects to isoniazid and 5 for resistance to isoniazid in the index case. There was no statistical significant difference in PT compliance between rifampicin and isoniazid (83.3% vs 62.7%; p=0.658).
DiscussionTuberculosis in childhood represents a missed opportunity for TB screening and establishment of PT.12 PT has the aim of precluding occurrence of disease in those already infected or exposed to TB. Despite its importance, there are some barriers, usually related with long PT courses and the lack of perception of the risk of TB development by the parents/caregivers in the asymptomatic child.2 The compliance to prolonged regimens is another difficult issue. In our study, initial compliance to PT was 63.9%, which was slightly inferior to another study that reported 72.8% of compliance to CP ant LTBI treatment in pediatric age.13 There was no statistical significant differences in the PT compliance between CP and LTBI patients (75.8% vs 53.8%, p=0.054), as also reported by Guix-Comellas et al.,13 which described an adherence of 24.3% by CP patients and 35.1% by LTBI patients, p=0.08, although with shorter regimens, young children on CP usually depend on their parents and are likely to adhere better to medical therapies. Older age was associated with non-compliance (p=0.046), consistent with another study that reported adolescence as a risk factor for non-compliance.13 Another study about treatment completion for LTBI reported 65.7% of treatment compliance, with significant higher adherence with 4-month rifampicin (85%) compared to isoniazid (52%).8 However, in our study no significant differences were found in the compliance between isoniazid and rifampicin (83.3% vs 62.7%; p=0.658), although the small number of patients on rifampicin may have limited the conclusions. In our population, isoniazid for 9 months was the chosen regimen, with 90% efficacy described in the literature.6,7,14 In cases in which Mt strains of the index case were resistant to isoniazid or intolerance to isoniazid was observed, a 4-month regimen with rifampicin was used, as described in the literature.6 Some studies suggest other shorter regimens with higher completion rates, such as 6-month therapy with isoniazid, with an efficacy of 69%,5 3–4 month of daily isoniazid plus rifampicin6 or twelve doses once-weekly with isoniazid and rifapentine, although this last regimen is not recommended for children younger than 2 years of age but has an estimated efficacy of 90%, equivalent to 9-months of isoniazid.6
The main barriers to PT implementation identified in different studies16,17 are, lack of awareness, lack of risk perception among parents, inadequate knowledge among healthcare providers and poor programmatic monitoring. However, in our study, social problems/family dysfunction and medication problems were the main reasons identified for non-compliance. We believe that our community-based approach with collaboration of pediatricians with experience in tuberculosis, with closer contact with families and regular scheduled appointments was responsible for an increased awareness of the health care providers to TB PT importance, reducing this non-compliance determinant reported in other studies. Another study in Ethiopia17 about compliance to isonazid CP reported poor compliance (12%) with the main reason being the perception that drugs were not necessary when the child was healthy.
Drug-related adverse effects were low, with just one patient needing to change medication. Routine liver function monitoring is not necessary for children unless they have liver disease10 and in our population they were performed in 33.4% of cases once during the treatment course. Household contacts were the most frequent source of infection, as also described by others.8,13
The implementation of several strategies was successful in the compliance improvement, achieving a final compliance of 98.6%. To the best of our knowledge, this is the first study in Portugal about PT compliance.
Our study has some limitations. First, its retrospective design and sample size limit the strength of the conclusions. Second, although this study considers a recruitment of participants at a community center, we cannot exclude the possibility of a selection bias. This may occur because some patients may not have been identified by public health services as tuberculosis contact patients and therefore were not included in our sample. Considering that this should represent a small number of patients, this bias is expected to have a minimal effect on the results. Finally, some factors found in other studies as determinants of compliance, such as parents’ education15 and cultural beliefs were not assessed in this study.
ConclusionsPT compliance was largely increased after implementation of improvement strategies. Non-compliance was associated with older age of patients. There was no significant difference in treatment compliance between rifampicin and isoniazid. A 9-month regimen with isoniazid continues to be the preferred modality for LTBI treatment. Compliance can be greatly improved by close monitoring and strategies to reconnect families with the PT, rather than shortening of treatment regimens. We emphasize the importance of health facilities inside the community, with experience in tuberculosis in children.
Conflict of interestThe authors declare that they have no conflict of interest.