Primary pleural lymphoma is a rare disease, with few cases reported in literature and corresponding to 2.4% of the primary thoracic wall tumors. Diffuse large B cell lymphoma (DLBCL) is the most common subtype reported, accounting for approximately 30% of non-Hodgking's lymphomas.1 Two types of primary pleural lymphomas were originally described in 1987, namely primary effusion lymphoma, principally associated to human immunodeficiency virus (HIV), and pyothorax-associated lymphoma, more related to human herpes virus-8 (HHV-8) infection and Ebstein-Barr virus (EBV) infection.2,3 Pathogenesis has not yet been elucidated but may be related to chronic inflammation of pleura surface that stimulates B-cells lymphocytes uncontrolled proliferation, resulting in pleural lymphoma development. Underlying pleural disease, such as tuberculous pyothorax or pneumothorax, is one of the suggested causes. Other reported cases are associated with some degree of immunosuppression and autoimmune diseases, as vasculitis, or even previous thoracic trauma.2,4 Clinical manifestations are unspecific, as the patient may present pleuritic chest pain, dyspnea, cough, fever, anorexia and weigh loss.4 The diagnosis is based on histopathological evidence, using thoracic surgery or thoracoscopy.5 The first choice of treatment is currently in discussion, but chemotherapy is the most frequently used.4 Anthracycline-based chemotherapies combined with anti-CD20 monoclonal antibody rituximab, as cyclophosphamide, pirarubicin, vincristine and prednisolone (CHOP) can significantly improve survival, with complete response rate of 30-45%.6 Cases of spontaneous remission are extremely rare, occurring with complete or partial resolution of the tumor without any treatment. The spontaneous remission mechanisms remain uncharacterized. A possible mechanism is the performance of pleural biopsy that generates a microtrauma and leads to an activation of pro-inflammatory state, alongside with enhanced immune recognition contributing to tumor control.7
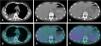
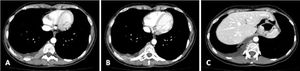
The authors report a clinical case of a 52-year-old female, smoker of 40 pack-years, teacher, with exposure to asbestos in her workplace. The patient had history of osteoporosis and three episodes of pneumonia in adolescence. No history of tuberculosis (TB) or recent contacts with TB. No previous thoracic trauma. No chronic medication. She was referred to pulmonology consultation due to one-month evolution of right posterior pleuritic chest pain which was progressively worsening, with no relief factors. She had no dyspnea, cough, fever or constitutional symptoms. Chest computed tomography (CT) scan revealed macronodular pleural thickening in lower half of right hemithorax with contrast hyperenhancement, associated to moderate right pleural effusion; lymph nodes in azygos-esophageal recess, the largest one with 17 mm diameter, and centrilobular emphysema in upper lobes (Fig. 1). Blood count revealed normocytic normochromic anemia (hemoglobin 11.6 g/dL, MGV 87.3 fL, MCH 31.4 pg). Auto-immune study was normal. There was no evidence of HIV infection. Respiratory functional study was normal. Transthoracic needle aspiration biopsy of pleural nodules was performed, and histopathological exam revealed diffuse pattern of malignant neoplasm extensively occupying the pleural surface, morphologically and immunophenotypically compatible with DLBCL. Microbiology analysis of pleural biopsy was negative, namely Mycobacterium tuberculosis (MTB). EBV-encoded ribonucleic acid was also tested in the biopsy, and it was negative. Positron emission tomography (PET) scan showed slight uptake of fluor-18-fluorodesoxyglucose (FDG) in pleural nodules and pleural effusion, with no other alterations (Fig. 2). Given the diagnostic doubt and the awareness of a rare entity, a surgical biopsy was performed seven weeks later. During the surgery, there was no evidence of pleural effusion and only a 3 mm nodular area was found and biopsied, showing pleural fragments extensively occupied by malignant neoplasia. Immunohistochemical study was positive for CD20, bcl-2, bcl-6 and MUM-1, and negative for CD10. These findings agreed with DLBCL diagnosis. The patient was referred to hematology consultation and the PET scan was repeated three months later, showing total absence of uptake FDG lesions. The patient improved clinically, with no chest pain and normalization of blood count. Complete spontaneous remission of primary pleural lymphoma was admitted. The patient is currently on clinical surveillance without recurrence of the disease after one year of follow-up, confirmed by PET scan.
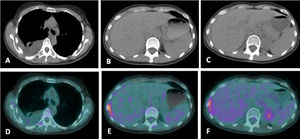
(A-F). PET scan and Chest CT scan showing slight uptake of fluor-18-fluorodesoxyglucose (FDG) in pleural nodules and pleural effusion (SUV max.=1.22); small area of pleural thickening between right liver lobe and 9th right rib with more intense FDG uptake (SUV max.=4.74); no other relevant alterations.
Pleural thickening or nodules associated with pleural effusion raised the hypothesis of an underlying neoplasm, namely pleural metastatic tumors or pleural mesothelioma, especially when exposures to asbestos is found, as in the present clinical case. Primary pleural DLBCL is a rare differential diagnosis of pleural nodules, particularly when there is no previous history of pleural inflammation, such as pneumothorax or pyothorax, immunosuppression, viral infection (HIV, EBV or HHV-8) or thoracic trauma. Clinical examinations such as imaging features and laboratory examination are unspecific. Therefore, an accurate histopathological diagnosis based on thoracic surgery or thoracoscopy is essential.
In conclusion, primary pleural lymphoma is a rare entity principally associated with chronic inflammatory stimulus. Chemotherapy is the most widely used treatment, with complete remissions rates of around 35%.2 On the other hand, spontaneous remissions are extremely rare.7 This case highlights the importance of primary pleural lymphoma in differential diagnosis of pleural disease, reinforcing the value of an accurate histological diagnosis.
Contributions of the authorsSSG and SC prepared the manuscript. TA and PR were responsible for patient management and revised the manuscript. HMS critically revised the manuscript and approved the final version. All authors read and approved the final manuscript.
None.