The long-term prognosis of patients with community-acquired pneumonia (CAP) has attracted increasing interest in recent years. The objective of the present study is to investigate the short and long-term outcomes in hospitalized patients with CAP and to identify the predictive factors associated with mortality.
Patients and methodsThe study was designed as a retrospective, multicenter, observational study. Hospitalized patients with CAP, as recorded in the pneumonia database of the Turkish Thoracic Society between 2011 and 2013, were included. Short-term mortality was defined as 30-day mortality and long-term mortality was assessed from those who survived 30 days. Predictive factors for short- and long-term mortality were analyzed.
ResultsThe study included 785 patients, 68% of whom were male and the mean age was 67±16 (18–92). The median duration of follow-up was 61.2±11.8 (37–90) months. Thirty-day mortality was 9.2% and the median survival of patients surviving 30 days was 62.8±4.4 months. Multivariate analysis revealed that advanced age, the absence of fever, a higher Charlson comorbidity score, higher blood urea nitrogen (BUN)/albumin ratios and lower alanine aminotransferase (ALT) levels were all predictors of long-term mortality.
ConclusionLong-term mortality following hospitalization for CAP is high. Charlson score and lack of fever are potential indicators for decreased long-term survival. As novel parameters, baseline BUN/albumin ratios and ALT levels are significantly associated with late mortality. Further interventions and closer monitoring are necessary for such subgroups of patients.
Community-acquired pneumonia (CAP) is a frequently observed, costly health issue causing significant morbidity and mortality. The incidence of CAP requiring hospitalization is about 25–30 cases per 10,000 adults and, in the USA, constitutes the seventh most frequent cause of all-cause death. Among infectious diseases, CAP is the most frequent cause of hospitalization and mortality in industrialized countries.1–3
In hospitalized patients, the mortality rate from CAP is around 10%.4–7 However, this rate varies depending on the hospital unit and the prognosis is worse for patients requiring treatment in an intensive care unit (ICU).
A large number of studies focus on the causal link between mortality and CAP, most of them analyzing in-hospital and short-term mortality.8,9 Fewer studies, however, have focused on the association between CAP and long-term mortality. At follow-up, pneumonia patients have displayed lower rates of survival and more frequent all-cause hospitalization, emergency department admissions and CAP-related visits compared to age and gender-matched control subjects without pneumonia.10 One-year mortality rate for patients with CAP is 17–40%, with increasing rates in the longer-term, independent of demographics and comorbid conditions.11–13 Recent work has sought to understand the reasons underlying the shorter survival of these patients and the incidence, factors, and predictors of long-term outcome. Long-term prognostic factors to be considered in CAP include advanced age, male gender, black race, health-care associated pneumonia, and chronic comorbid illnesses.5 To date, only a few systematic data evaluating long-term outcomes for these patients have been reported.
The objective of the present study is to investigate the short and long-term outcome in hospitalized patients with CAP and to identify the predictive factors of mortality.
MethodsThis study was designed as a retrospective, multicenter, observational cohort study and was conducted in eight hospitals (seven university hospitals and one training and research hospital) within Turkey. The study complied with the ethical principles of the Declaration of Helsinki and was approved by the local Ethics Committee.
Study population and data collectionWe included patients diagnosed with CAP between 2011 and 2013, from all centers around the country. Data have been collected from the Turkish Thoracic Society pneumonia database (TURCAP: Turkey Community Acquired Pneumonia) and hospitalized patients with CAP and an available identity number were included in the study.
Patients were diagnosed with CAP if pulmonary infiltration was visible in a chest X-ray and if there were clinical symptoms such as a cough, purulent sputum, pleuretic chest pain, shortness of breath, fever or findings detectable by auscultation.14 Patients under 18 years of age, with no registered identity number or having been managed in outpatient clinics, were excluded from the study.
Baseline demographics, symptoms, physical examination findings and vital signs at presentation, smoking status, and comorbid conditions were recorded. Charlson comorbidity score was calculated for each patient.15,16 Laboratory data included white blood cell count (WBC), and levels of blood glucose, BUN, ALT, albumin and C-reactive protein (CRP). BUN/albumin ratios were calculated.14 Chest X-ray findings (i.e. alveolar consolidation, interstitial pattern, pleural effusion, lobar or multilobar involvement) were included. The severity of illness scores of CURB-65 (confusion, uremia, respiratory rate, low blood pressure, age 65 years or older) and PSI (pneumonia severity index) was recorded.17
Analysis of mortalitySurvival status of the patients was assessed via identity numbers using the National Death Certificate Database (www.obs.gov.tr). The 30-day, 90-day, and 1–5 year mortality were calculated for every patient from the day of the presentation. Short-term mortality was defined as 30-day mortality from admission. For the analysis of long-term mortality, those who survived the initial acute event were included and all-cause long-term mortality until the completion of follow-up was assessed.
Statistical analysisQuantitative data are expressed as mean±standard deviation (SD) and qualitative data are expressed as frequencies. In-hospital mortality was evaluated using Student's t-test and chi-square tests. All-cause mortality was reported for long-term follow-up. Survival curves were drawn using the Kaplan–Meier method. Cox's proportional hazards model was used to determine the potential predictors of mortality. Independent variables associated with mortality with a p value ≤0.05 in the univariate analysis were then incorporated into a multivariate analysis, also based on Cox's proportional model. All statistical analyses were conducted using a statistical software package (SPSS for Windows, version 16.0; SPSS Inc.; Chicago, IL, USA). A p value of ≤0.05 was considered to be significant.
ResultsOf all the 785 patients included in the study, 68% were male and the mean age was 67±16 (18–92). A cough, sputum, and fever were the most common presenting symptoms. Overall, 82% of the patients had been diagnosed with at least one additional disease including: chronic obstructive pulmonary disease (COPD), asthma, congestive heart failure (CHF), coronary heart disease (CHD), diabetes mellitus (DM), malignancy, chronic renal disease (CRD), chronic liver disease and cerebrovascular disease. The average PSI and CURB-65 scores were 99.1±35.9 (15–243) and 2.16±0.98 (0–5), respectively.
Radiological findings included alveolar consolidation in 73% of the patients and pleural effusion in 21%. Multilobar infiltration was noted in 29% of patients (Table 1).
Baseline characteristics and comparison of patients according to 30-day mortality.
| All patients | Non-survivors | Survivors | p | |
|---|---|---|---|---|
| N=785 | N=72 (9.2%) | N=713 (90.8%) | ||
| Demographics | ||||
| Gender N (%) | ||||
| Female | 253 (32) | 26 (36) | 227 (32) | 0.509 |
| Male | 532 (68) | 46 (64) | 486 (68) | |
| Age, mean (years) | 67±16 | 75±13.0 | 66±17 | <0.0001 |
| Ever smoker (N %) | 504 (64) | 41 (57) | 463 (65) | 0.114 |
| Smoking load (pack/year) | 40±34 | 41±26 | 40±34. | 0.848 |
| Charlson comorbidity score | 1.89±1.74 | 2.61±2.1 | 1.8±1.7 | <0.0001 |
| Laboratory values | ||||
| WBC (×109/L) | 13.5±7.6 | 12.3±5.5 | 13.6±7.8 | 0.209 |
| Hematocrit (%) | 37.1±6.3 | 35.0±6.5 | 37.2±6.3 | 0.005 |
| Glucose (mg/dL) | 146±80 | 158±73 | 145±81 | 0.206 |
| ALT (U/L) | 29.5±28.0 | 27.4±23.1 | 29.7±28.4 | 0.634 |
| BUN/albumin | 9.4±8.8 | 15.1±14.8 | 8.9±8.1 | 0.001 |
| CRP (mg/L) | 50.3±79.9 | 66.7±99.1 | 49.2±78 | 0.230 |
| PaO2 (mmHg) | 60.3±13.9 | 60.4±11.7 | 60.3±14.2 | 0.964 |
| CXR (N, %) | ||||
| Consolidation | 571 (73) | 56 (78) | 515 (72) | 0.405 |
| Interstitial infiltration | 263 (34) | 20 (28) | 243 (34) | 0.298 |
| Abscess | 8 (1) | 0 | 8 | 0.999 |
| Pleural effusion | 161 (21) | 23 (32) | 138 (19) | 0.020 |
| Multilobar infiltration | 212 (29) | 28 (42) | 184 (27) | 0.023 |
| PSI score | 99.1±35.9 | 137.0±35.3 | 95.2±33.7 | <0.0001 |
| CURB-65 score | 2.16±0.98 | 3.0±1.16 | 2.08±0.92 | <0.0001 |
ALT, alanine aminotransferase; BUN, blood urea nitrogen; CRP, C-reactive protein; CXR, chest X-ray; PSI, pneumonia severity index; WBC, white blood cell.
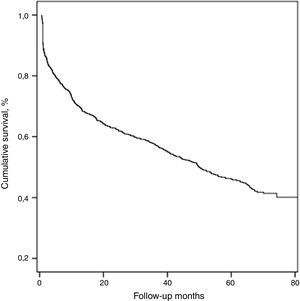
The median duration of follow-up was 61.2±11.8 (37–90) months. During this period, 428 (54.5%) patients died and the median survival of all patients was 50.1±4.2 months.
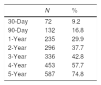
With respect to all-cause mortality, 30-day and 90-day mortality were 9.2% and 16.8%, respectively. Cumulative percentage mortality from one to five years post admission was 29.9%, 37.7%, 42.8%, 57.7%, and 74.8%, respectively (Table 2, Fig. 1).
The risk of death increased significantly with higher PSI and CURB-65 scores and Charlson comorbidity score. Neither smoking status nor cigarette smoking load (pack/year) affected the short-term outcome. Among baseline laboratory parameters; lower hematocrit levels and higher BUN/albumin ratios predicted 30-day mortality. Similarly, a significantly worse short-term outcome (p<0.05) was seen in the presence of pleural effusion and multilobar involvement (Table 1).
Long-term mortalityLong-term mortality was assessed in 713 patients who had survived longer than 30 days after presentation. The median survival of this cohort of patients was 62.8±4.4 months.
In univariate analysis; male gender, advanced age, having ever smoked and heavier smoking, were the parameters associated with mortality (p<0.05). The late mortality rate was increased in patients with a higher Charlson comorbidity score and in the absence of fever. Among baseline laboratory parameters, lower levels of ALT and higher BUN/albumin ratios were predictors of increased long-term mortality. PSI and CURB-65 scores were also found to be related to long-term mortality (Table 3).
Univariate analysis of the factors related to long-term mortality (Cox proportional hazard model).
| HR | CI (95%) | p | |
|---|---|---|---|
| Demographics | |||
| Male gender | 0.654 | 0.516–0.829 | <0.0001 |
| Advanced age | 1.038 | 1.031–1.044 | <0.0001 |
| Ever smoking | 0.711 | 0.562–0.899 | 0.004 |
| Smoking load (pack/year) | 1.004 | 1.002–1.007 | 0.003 |
| Presenting symptoms | |||
| Absence of fever | 1.757 | 1.427–2.164 | <0.0001 |
| Cough | 1.091 | 0.801–1.488 | 0.580 |
| Sputum | 0.830 | 0.625–1.101 | 0.196 |
| Charlson comorbidity score | 1.209 | 1.154–1.266 | <0.0001 |
| Laboratory values | |||
| WBC (×109/L) | 1.000 | 1.000–1.000 | 0.236 |
| Hematocrit (%) | 0.988 | 0.972–1.005 | 0.172 |
| Glucose (mg/dL) | 1.000 | 0.998–1.001 | 0.542 |
| Lower ALT (U/L) | 0.988 | 0.981–0.995 | 0.001 |
| Higher BUN/albumin | 1.049 | 1.035–1.064 | <0.0001 |
| CRP (mg/L) | 1.001 | 0.999–1.003 | 0.284 |
| PaO2 (mmHg) | 0.993 | 0.982–1.003 | 0.158 |
| CXR (N %) | |||
| Consolidation | 0.917 | 0.721–1.166 | 0.479 |
| Interstitial infiltration | 1.190 | 0.948–1.494 | 0.133 |
| Abscess | 2.186 | 0.544–8.774 | 0.270 |
| Pleural effusion | 0.805 | 0.625–1.038 | 0.094 |
| Multilobar infiltration | 1.060 | 0.932–1.206 | 0.376 |
| Higher PSI score | 1.019 | 1.016–1.022 | <0.0001 |
| Higher CURB-65 score | 1.579 | 1.422–1.754 | <0.0001 |
ALT, alanine aminotransferase; BUN, blood urea nitrogen; CRP, C-reactive protein; CXR, chest X-ray; PaO2, partial arterial oxygen pressure; PSI, pneumonia severity index; WBC, white blood cell.
Multivariate analysis included gender, age, smoking status, Charlson comorbidity score, lack of fever, ALT and BUN/albumin ratios. Independent factors predicting long-term mortality were advanced age, Charlson comorbidity score, absence of fever, higher BUN/albumin ratios and lower ALT levels (Table 4).
Multivariate analysis of the factors related to long-term mortality (Cox-proportional hazard model).
| HR | CI (95%) | p | |
|---|---|---|---|
| Male gender | 0.728 | 0.456–1.161 | 0.182 |
| Age | 1.029 | 1.017–1.041 | <0.0001 |
| Ever smoking | 0.917 | 0.585–1.438 | 0.706 |
| Charlson comorbidity score | 1.180 | 1.081–1.289 | <0.0001 |
| Absence of fever | 0.558 | 0.398–0.783 | 0.001 |
| BUN/albumin | 1.035 | 1.018–1.052 | <0.0001 |
| Lower ALT | 0.990 | 0.981–0.999 | 0.026 |
ALT, alanine aminotransferase; BUN, blood urea nitrogen.
The present study confirms the increased mortality rates following pneumonia. In terms of short-term mortality, aside from PSI and CURB-65 scores, higher ratios of serum BUN/albumin and radiological multilobar involvement were found to be significant predictors of a worse prognosis. Independent predictors for long-term mortality were advanced age, Charlson comorbidity score, absence of fever, higher BUN/albumin ratios and lower ALT levels. BUN/albumin ratios and ALT levels in this large study group are described for the first time as primary predictors of long-term outcome.
The 30-day mortality of hospitalized patients with CAP was verified by this study to be approximately 8.7–12%.7,8,18 Long-term survival rates vary widely in the literature. One-year mortality is reported to be between 17% and 40.9%.11,13 Two-year, 3-year and 5-year mortalities are reported as 27%, 31%, and 43%, respectively.13,18,19 Johnstone et al. reported 3.8-year mortality as 53%.7 Such variability could be related to different study groups, and/or exclusion of short-term mortality patients from the long-term mortality rate analysis. The current study calculated mortality rates for all the patients and has found survival rates to be low, in common with the existing literature.
The absence of fever at presentation was found to have a significant impact on mortality. The presence of fever had also been found to be protective in other studies.20 PORT study concluded that the lack of feeling feverish was related to poorer outcome compared to age-matched controls.18 We believe that infections causing fever reflect a better immune response and for effective host defense, hence leading to a better outcome. Higher respiratory rates may be related to more severe pneumonia. The relationship between long-term survival and respiratory rate would benefit from further research.
Previous studies evaluating causes of death have shown that the absolute CAP-related mortality rate is low whereas malignancy and diseases of the circulatory and respiratory systems are the most common causes of death.10,13 At follow-up, COPD, malignancy, neurodegenerative disorders and cardiovascular diseases were shown to increase mortality.5,13,20–22 Charlson index score has been correlated independently with the long-term outcome.18,23 Similarly, in the current study, Charlson score was found to be an independent predictor of long-term mortality. Furthermore, the score was also found to be a predictor of short-term outcome.
Lower albumin and higher levels of serum urea have been shown to increase mortality.24,25 Elevated serum BUN/albumin ratio was firstly described as a predictor of 28-day mortality by Ugajin et al. They also concluded that BUN/albumin ratio predicted the requirement for intensive care treatment in CAP.14 Contrary to this study, Akpinar et al. found that this ratio was able to predict the ICU admission but not 30-day mortality.26 However, the sample size was small in both of these studies. In the current study, a higher BUN/albumin ratio predicted a worse 30-day outcome. Additionally, this ratio predicted long-term survival in an independent manner. To the best of our knowledge, BUN/albumin ratio is described as a predictor of long-term prognosis for the first time.
Previous studies have shown that abnormal liver function tests, particularly raised ALT, increase in-hospital mortality.7,27 However, in the present study, no such relationship was discovered between liver function tests and short-term survival. Conversely, higher levels of ALT were protective for long-term survival. To the best of our knowledge, liver function tests have not been evaluated in terms of long-term outcome. Further studies are needed to cast light on this relationship.
In accordance with the literature, PSI and CURB-65 scores were found to be predictors of short-term mortality.14,28 PSI and CURB-65 scores have also been reported to be predictors of long-term mortality.18,28
The following limitations to this paper should be noted. First, it was a retrospective study. Second, an analysis of cause of death was not performed. Third, outpatients and ICU admissions were not included in the study. The strength of this study, on the other hand, is the large study sample with a well-documented database. Additionally, it is a multicenter study reflecting results which geographically covered the whole country.
In conclusion, long-term mortality following hospitalization for CAP is high. Patients with advanced age, higher Charlson score, higher respiratory rates and lower fever have a significantly increased chance of late mortality. Baseline BUN/albumin ratios and ALT levels are significant predictors of survival. Further interventions and closer monitoring would be necessary for such patient subgroups.
Conflict of interestThere is no conflict of interest related to this paper. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Informed consent was obtained from the patients included in the TURCAP database.
We kindly thank to Turkish Thoracic Society for TURCAP pneumonia database.