Giant Cell Arteritis (GCA), also known as “Temporal Arteritis” or “Horton's disease”, is a common systemic vasculitis that causes inflammation of large and medium-sized arteries, especially the temporal artery.1–3 The pathogenesis is not well known but has been linked to an autoimmune process.3 It is rare in individuals younger than 50 years1,2,4 and incidence increases with age.3 It affects women more often than men.2,3
Its most serious complication is visual loss which is almost always permanent when established but can be prevented by rapid treatment.2,3 Other complications include stroke, myocardial infarction2 and aortic aneurism formation.3
Diagnosis is considered based on medical history, clinical evaluation, and laboratory and imaging tests. It is confirmed by histological findings.3 In the absence of typical signs and symptoms, diagnosis becomes difficult.4 Blood tests can be useful, specifically elevated acute phase reactants such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP).2,3,5 The same tests may be used to evaluate the response to treatment.5 A cranial ultrasound may provide additional clues but arterial biopsy is still the gold standard for making a definitive diagnosis.2
GCA may rarely present with pulmonary or pleural involvement. In this article, the authors report a rare case of histologically proven GCA with pulmonary nodules.
Case reportA 74-year-old man was observed at the emergency department for low grade fever (38°C), bilateral headaches and blurred vision of the left eye, which had started 10 days earlier. He also complained of general malaise, anorexia, weight loss of 4kg, muscle pain predominating in upper limbs and bilateral shoulder pain and jaw claudication within the last 2 months. He had neither respiratory nor genitourinary tract complaints.
He had a history of pulmonary tuberculosis at the age of 13, Sydenham's chorea, epilepsy, granulomatous dermatitis, recurrent uveitis of both eyes and post-phacoemulsification of left eye. He was chronically treated with bisoprolol and carbamazepine. He was a former smoker (until twenty years earlier).
On physical examination during admission he was afebrile, skin and mucosae were pale, and the left temporal artery was tortuous, thickened and tender. The remaining physical examination revealed no abnormal findings. The patient was promptly observed by ophthalmology, having been diagnosed with anterior uveitis of the left eye.
Laboratory tests showed a normal leucogram with a marked increase in acute phase reactants with a CRP of 15.90mg/l (normal range: <0.50) and ESR of 117mm in the first hour (normal range: 0.0–10.0); normochromic normocytic anaemia with haemoglobin of 9.6g/dl (normal range: 14.0–18.0) and elevated liver enzymes with gamma-glutamyl-transferase (GGT)=546.9IU/l (normal range: 7–49), alkaline phosphatase (AF)=297IU/l (normal range: 25–100), alanine-amino-transaminase (ALT) 89=IU/l (normal range: 4–43) and aspartate-amino-transaminase (AST)=49IU/l (normal range: 4–43) with normal bilirubin values. Renal function and urinalysis were normal.
Given the high diagnostic suspicion of GCA the patient was immediately put on systemic corticotherapy on a prednisolone dose of 1mg/kg/day combined with topical ocular steroid.
The patient was admitted to hospital and given a diagnostic workup. Abdomen ultrasound (USS) showed signs of hepatic steatosis and a poorly defined nodular area in the IV segment. No abnormalities could be detected on chest X-ray.
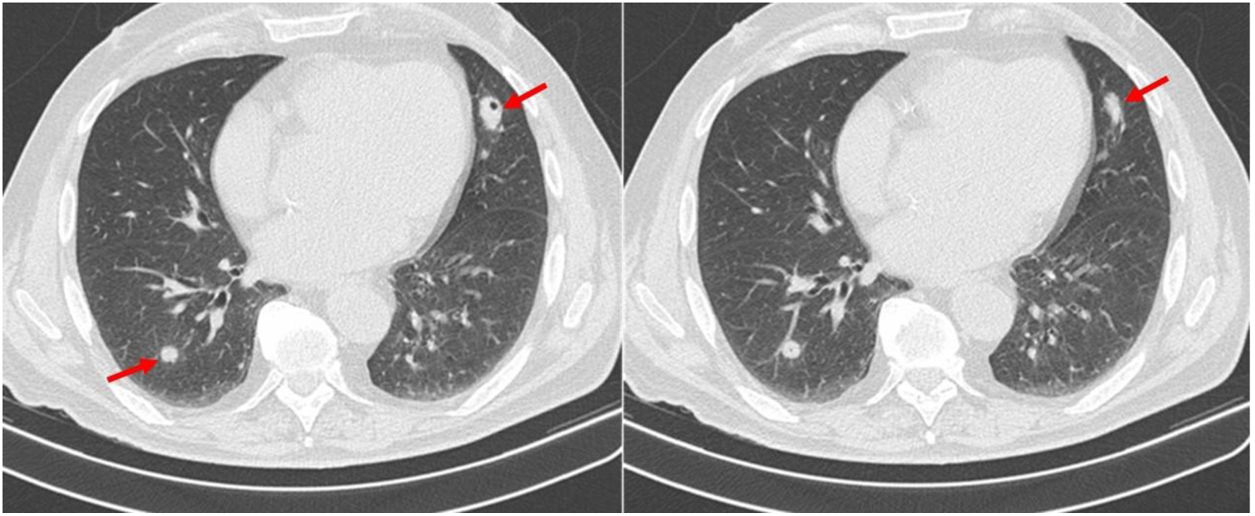
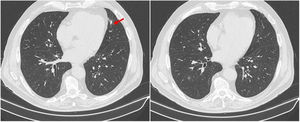
Due to accompanying symptoms of general malaise, anorexia, weight loss and considering the hepatic nodule, a computed tomography (CT) of chest, abdomen, and pelvis was performed. It showed that the poorly defined nodular area in the IV liver segment corresponded to focal hepatic steatosis and identified four pulmonary nodules, three of them cavitary. The largest nodule was located in the inferior lingular segment, measuring 24×17mm and with spiculated contours (Fig. 1). Neither bronchoscopy nor bronchoalveolar lavage (performed on right middle lobe) showed relevant alterations. Cytological examination of bronchial aspirate was negative for malignant tumour cells, and culture was negative for pathogens including mycobacteria and fungi.
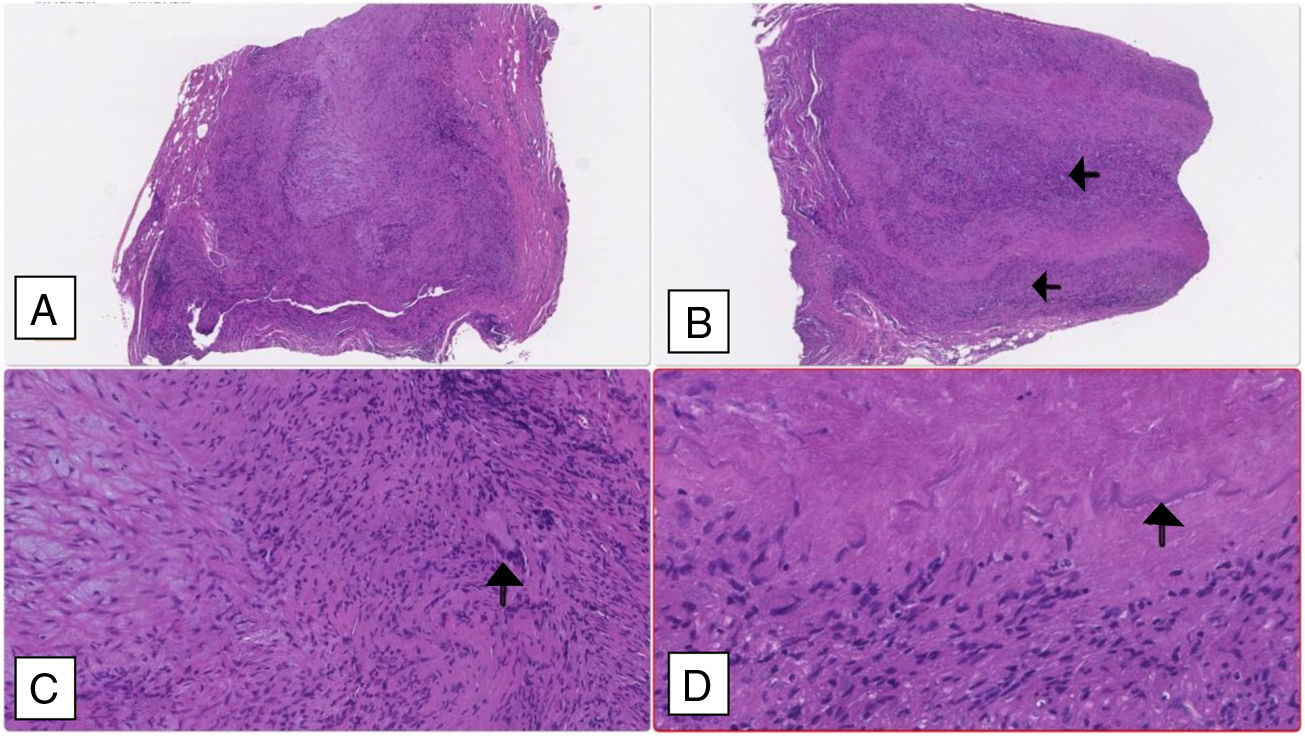
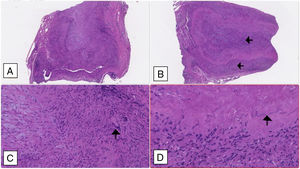
A temporal artery biopsy was performed four days after introduction of steroid therapy and showed features compatible with a GCA diagnosis (Fig. 2).
Temporal artery biopsy: (A and B) Cross section of arterial wall (haematoxylin–eosin 2.5×) showing luminal obliteration and more intense lympho-histiocytic inflammatory infiltrate in the intima and middle tunica (black arrows). (C) Multinucleated giant cell marked with a black arrow (haematoxylin–eosin 10×); (D) fragmented inner elastic blade (black arrow).
A transthoracic lung biopsy was performed. The result of lung biopsy revealed unspecific chronic mononuclear inflammatory infiltrate. Giant cells were not found in the sample. No granulomas, angeitis, necrosis or eosinophils were noted. Nuclear atypia was not present and no microorganisms were seen.
Later we obtained other blood tests results, namely serum antineutrophil cytoplasmic antibodies (ANCAs), serum angiotensin converting enzyme (SACE), rheumatoid factor (RF) and antinuclear antibodies (ANAs), all of them negative.
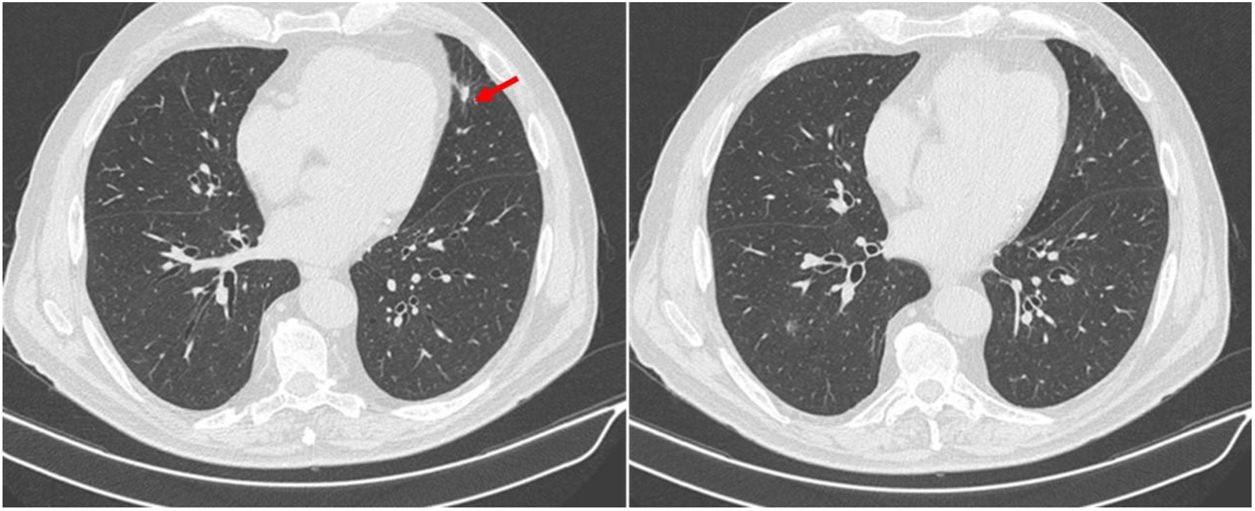
After clinical and analytical improvement and resolution of all the complaints, including visual improvement, the patient was discharged on prednisolone 1mg/kg/day and acetylsalicylic acid 100mg/day. The prednisolone dose was tapered over two months to 60mg/day, maintaining symptom control and stable blood work. At this time, chest CT was repeated and showed resolution of the pulmonary nodular lesions (Fig. 3).
One year after discharge, the patient remains clinically asymptomatic. However, the CPR ranged from 1.8 to 2.8mg/dl and he presented an inflammatory anaemia with prednisolone dose higher than 7.5mg/day. Methotrexate 15mg/week was associated. Later, a whole body positron emission tomogram (PET) showed activity of vasculitis with involvement of large vessel, so we initiated tocilizumab.
DiscussionThe diagnosis of GCA was not difficult in our patient because it presented with typical symptoms and signs, and met all the classification criteria of GCA as proposed by the American College of Rheumatology.6 The patient was over 50 years of age, presented with new onset headaches and demonstrated temporal artery tenderness at palpation. He also had jaw claudication, which is a typical symptom of the disease and symptoms compatible with polymyalgia rheumatic.2,5,7 Polymyalgia Rheumatica is observed in more than one-half of patients with GCA.1 Associated constitutional symptoms including fatigue, malaise, weight loss and anorexia, low-grade fever were also described.2,3,5,7
The patient had decreased visual acuity at presentation. Visual manifestations are usually among the presenting symptoms. They may also develop shortly after the diagnosis in nearly 30% of patients and range from transient diplopia or amaurosis fugax to sudden unilateral or bilateral partial or complete visual loss.2,3 Uveitis is a rare form of presentation.8
The patient had elevation of the inflammatory biomarkers, namely ESR greater than 50mm/h, and responded rapidly to corticosteroid treatment. He had mild anaemia and elevated serum concentrations of hepatic enzymes such as aspartate aminotransferase and alkaline phosphatase, which can occur in 25–35% of patients.2,5
Due to the high clinical suspicion and the impossibility of immediate biopsy prompt corticosteroid therapy was initiated. Although biopsy was made after four days of corticosteroid, histological features remained interpretable for at least two weeks after starting treatment.3 Histopathological characteristics of the temporal biopsy showed vasculitis with a predominance of mononuclear-cell infiltration and multinucleated giant cells which is one of the 5 classification criteria of GCA proposed by the American College of Rheumatology.6
The presence of pulmonary nodules is not a common feature of GCA. Lung biopsy helped to exclude Granulomatosis with Polyangiitis (GPA), infection and malignancy. In our patient ANCAs were negative. This does not exclude the possibility of GPA but does makes the diagnosis less likely.1 In addition, the absence of respiratory symptoms, renal involvement and granulomas in the lung biopsy, along with the prompt response to corticosteroid therapy, are more suggestive of GCA rather than GPA. GPA response to therapy usually occurs over weeks to months and generally does not respond to corticosteroid therapy alone, usually requiring immunosuppressive agents, notably cyclophosphamide.9
Infectious diseases, especially those provoked by mycobacteria and fungi, may cause granulomas with necrosis. This was not detected in the lung biopsy of our patient. Cultures from the bronchoalveolar lavage were also negative.
The biopsy showed no malignant cells. The rapid disappearance of the pulmonary nodules with corticosteroid therapy was also an argument against malignance.
CT-guided lung biopsy showed unspecific inflammatory histological features. In fact, no giant cells were found. However, a GCA diagnosis does not require the demonstration of giant cells3,10; giant cells are detected in only 60–70% of histological samples.2 GCA is a systemic vasculitis. We consider the pulmonary nodules to be a systemic manifestation of the disease.11 Involvement of the respiratory system has been reported with a frequency ranging from 9% to 31% of GCA patients.4 Respiratory symptoms described include cough,9,11,13 dyspnoea,11 sore throat, hoarseness, choking sensation and thoracic pain.11,12 Few cases of GCA associated with lung involvement have been published, those with, for example, alveolar haemorrhage,12–15 pleural effusion4,11–13,16–18 and interstitial pulmonary disease7,11,13,19,20 and pulmonary nodules are rare.7,9,11,13,20,21
Our patient started corticosteroid therapy promptly, as recommended. Glucocorticoids are the mainstay of therapy and rapid response to corticosteroid is also typical of GCA.2,3 After successful control of the disorder, the dose of corticosteroids can be reduced with careful monitoring using both clinical and laboratory parameters to assess relapse. There is mixed evidence for the use of adjunctive corticosteroid-sparing agents such as methotrexate.3 Aspirin (100mg daily) could decrease the rate of vision loss and stroke during the course of the disease.3
In many patients GCA can be controlled and resolved in a few months to years. Most do not require prednisolone therapy after 12–24 months. A minority of patients will need a small dose to control symptoms for many years.3 Patients should be followed closely in case of relapse, large vessel involvement and steroid-related complications.3 Our patient is still under regular surveillance.
ConclusionsFew cases of GCA associated with lung involvement have been published. It is important to recognize these atypical presentations because they may be the sole initial manifestation of the disease.11 GCA is eminently treatable when promptly diagnosed. A delayed diagnosis may lead to potentially devastating consequences.3,11 This emphasizes the need for early diagnosis. Corticosteroid treatment should not be withheld while awaiting the results of a temporal artery biopsy, which remains the gold standard for GCA diagnosis.3
Conflicts of interestThe authors have no conflicts of interest to declare.
It was discovered that the original online version of the above article was incorrectly assigned to the Case Report section. The Publisher decided to replace the XML and the online PDF of the article in its correct formatted version as a Research Letter. The printed issue has also been correspondingly altered.
The Publisher informs that the content of the manuscript hasn’t been transformed and only the secondary changes related to the change of layout have been included.
Elsevier regrets and apologizes for any inconvenience caused by posting a new version of this article online and hopes that the reader will understand the reasons for doing so.