Drug resistant tuberculosis (TB) is the result of a selective pressure on the Mycobacterium tuberculosis, which has been in action, over the last 70 years.
One of the major threats to the control of TB is posed by the development and spread of multidrug-resistant strains (MDR-TB) that are resistant to at least isoniazid (INH) and rifampicin (RMP), the two most powerful anti-TB drugs currently available; this is often the result of their misguided use and poor investment in control programs.1,2 These patients eliminate bacillus for a longer period, which increases the dissemination risk of this disease, posing an alarming problem for Public Health. Their treatment is more difficult to manage due to increased morbidity and higher costs.
This growing concern led to the development of this study, in order to assess the resistance patterns to first line anti-TB drugs among pulmonary (respiratory) TB cases notified and residing in the Coimbra District (Portugal) between 2000 and 2011 and to determine among them the risk factors for MDR-TB.
The study was designed as a retrospective cohort one. It was carried out at the Respiratory Diagnostic Centres of Coimbra and Figueira da Foz. 1057 TB cases were reviewed. Pulmonary TB was the most frequently reported diagnostic site (744/1057), of which 556 cases resided in Coimbra District. Clinical records were reviewed, in order to analyze data on all verified culture positive Mycobacterium tuberculosis complex (MTC) cases along with drug susceptibility testing (DST). Although 428 cases were registered as culture-positive, of which 389 cases had MTC registered on their files, only 339 had a registered reference (clinical or laboratory archive) to DST for first-line drugs (study population). There were 15 cases diagnosed when treated with a full course of anti-TB treatment, 45 exclusively with a biopsy, and 157 DST results unavailable and/or unregistered due to technical and administrative reasons. The adjusted proportion of cases with registered DST was of 68.3% (339/496). Ultimately the study population was affected by a selection bias, typical of retrospective studies.
The first line anti-TB drugs considered for this study were: INH, RMP, ethambutol (EMB), pyrazinamide (PZA) and streptomycin (SM).
There were six study variables: socio-demographic, co-morbidities, risk groups (alcohol, tobacco smoke and/or drug abuse, residents in community shelters, homeless, and prisoners), previous treatment history (for disease or latent infection), drug susceptibility result, and resistance pattern. In order to determine independent risk factors for MDR pulmonary TB, categorical data were compared by the chi-square test or the fisher exact test. A p value of less than 0.05 was considered as statistically significant.
The study population enrolled 73.2% (248/339) male patients. The median age of the patients was 43 years, with a minimum and maximum of 18 and 90 years, respectively.
HIV screening status was registered in only 32.1% (109/339) of the patients selected. Of those 109 patients, 25 were HIV positive, of whom 8 were foreign-born.
Considering the difficulty of growth-based testing for PZA, its DST was unavailable in 5.5% (19/339) of the cases. There was one case without registered DST for SM (Table 1).
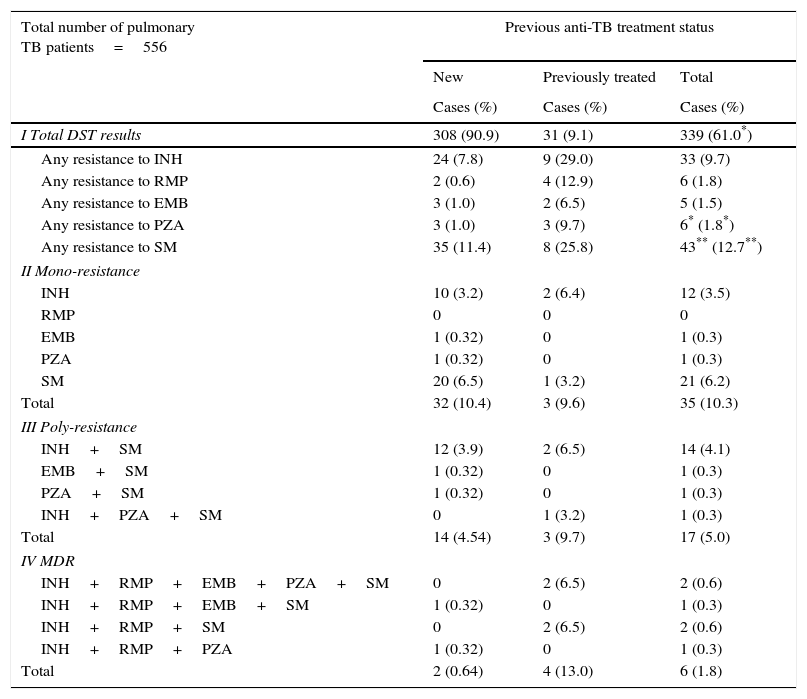
First line anti-tuberculosis drug resistance profile in new and previously treated patients.
| Total number of pulmonary TB patients=556 | Previous anti-TB treatment status | ||
|---|---|---|---|
| New | Previously treated | Total | |
| Cases (%) | Cases (%) | Cases (%) | |
| I Total DST results | 308 (90.9) | 31 (9.1) | 339 (61.0*) |
| Any resistance to INH | 24 (7.8) | 9 (29.0) | 33 (9.7) |
| Any resistance to RMP | 2 (0.6) | 4 (12.9) | 6 (1.8) |
| Any resistance to EMB | 3 (1.0) | 2 (6.5) | 5 (1.5) |
| Any resistance to PZA | 3 (1.0) | 3 (9.7) | 6* (1.8*) |
| Any resistance to SM | 35 (11.4) | 8 (25.8) | 43** (12.7**) |
| II Mono-resistance | |||
| INH | 10 (3.2) | 2 (6.4) | 12 (3.5) |
| RMP | 0 | 0 | 0 |
| EMB | 1 (0.32) | 0 | 1 (0.3) |
| PZA | 1 (0.32) | 0 | 1 (0.3) |
| SM | 20 (6.5) | 1 (3.2) | 21 (6.2) |
| Total | 32 (10.4) | 3 (9.6) | 35 (10.3) |
| III Poly-resistance | |||
| INH+SM | 12 (3.9) | 2 (6.5) | 14 (4.1) |
| EMB+SM | 1 (0.32) | 0 | 1 (0.3) |
| PZA+SM | 1 (0.32) | 0 | 1 (0.3) |
| INH+PZA+SM | 0 | 1 (3.2) | 1 (0.3) |
| Total | 14 (4.54) | 3 (9.7) | 17 (5.0) |
| IV MDR | |||
| INH+RMP+EMB+PZA+SM | 0 | 2 (6.5) | 2 (0.6) |
| INH+RMP+EMB+SM | 1 (0.32) | 0 | 1 (0.3) |
| INH+RMP+SM | 0 | 2 (6.5) | 2 (0.6) |
| INH+RMP+PZA | 1 (0.32) | 0 | 1 (0.3) |
| Total | 2 (0.64) | 4 (13.0) | 6 (1.8) |
Among those enrolled, 17.1% were resistant to at least one anti-TB drug. The drug resistance observed for any of the first line drugs in new and previously treated patients was 7.8% and 29% to INH, 0.6% and 12.9% to RMP; 1% and 6.5% to EMB; 1% and 9.7% to PZA; 11.4% and 25.8% to SM, respectively (Table 1).
The cases resistant to at least one first line anti-TB drug peaked by 32.3% (10/31) in 2006. Those susceptible to all first-line anti-TB drugs peaked by 88.5% (23/26) in 2009. The resistance to INH and RMP peaked in 2006, with 19.4% (6/31) and 6.5% (2/31), respectively; to EMB peaked in 2011, with 10% (2/20); to PZA peaked in 2003, with 8.6% (3/35); and to SM in 2006, with 29% (9/31). There were six patients identified with MDR pulmonary TB over the study period; with the highest incidence of 6.9% (2/31) in 2006.
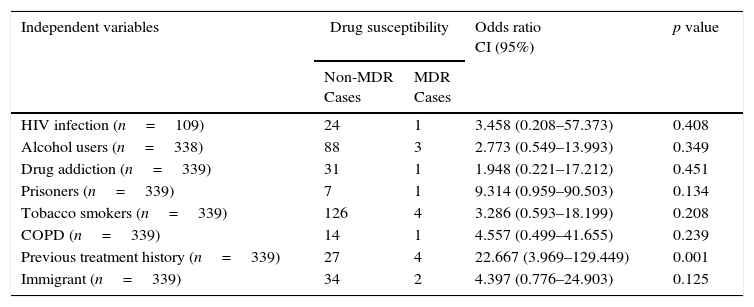
Resistance to PZA was reported in 0.9% (3/314) and 50% (3/6) of non and MDR-TB cases, respectively. The association of MDR-TB with previous treatment history was significant with an odds ratio of 22.7 (95% CI; 3.9–1294; p-value, 0.001) (Table 2).
MDR-TB risk factors among pulmonary TB cases in Coimbra District, 2000–2011.
| Independent variables | Drug susceptibility | Odds ratio CI (95%) | p value | |
|---|---|---|---|---|
| Non-MDR Cases | MDR Cases | |||
| HIV infection (n=109) | 24 | 1 | 3.458 (0.208–57.373) | 0.408 |
| Alcohol users (n=338) | 88 | 3 | 2.773 (0.549–13.993) | 0.349 |
| Drug addiction (n=339) | 31 | 1 | 1.948 (0.221–17.212) | 0.451 |
| Prisoners (n=339) | 7 | 1 | 9.314 (0.959–90.503) | 0.134 |
| Tobacco smokers (n=339) | 126 | 4 | 3.286 (0.593–18.199) | 0.208 |
| COPD (n=339) | 14 | 1 | 4.557 (0.499–41.655) | 0.239 |
| Previous treatment history (n=339) | 27 | 4 | 22.667 (3.969–129.449) | 0.001 |
| Immigrant (n=339) | 34 | 2 | 4.397 (0.776–24.903) | 0.125 |
HIV – human immunodeficiency virus; COPD – chronic obstructive pulmonary disease;
p – chi-square test or the fisher exact test.
The overall resistance to SM (12.7%) was the most prevalent among the first line anti-TB drugs. It might be due to its prolonged availability that dates from 1940s, which has lead to the development of resistant strains.3
PZA is an important component of TB treatment, having remarkable sterilizing effects in killing semidormant TB bacilli. Its resistance pattern was similar to that reported in the United States (2.2% and 38% of non and MDR-TB cases, respectively).4
The prevalence of strains with primary resistance to INH is a very important indicator to estimate the risk of development of MDR.3,5 Over the study period, its value was of 7.8%, below the European Union average (8.1%).3 However, resistance to INH among previously treated patients was the most prevalent (29%).
The overall resistance to RMP has a high positive predictive value to MDR, once all cases are MDR-TB.
In Portugal, the incidence of MDR-TB has been decreasing, representing in 2009, 1.5% of the total cases of TB.6 Coimbra District is one of the regions with the lowest incidences of the disease; there were no notifications of MDR-TB cases6 between 2009 and 2011.
Previous treatment was recognized as the strongest risk factor for MDR-TB (Table 2), which is also observed in Europe.7
Delayed recognition of drug resistance, inappropriate chemotherapy regimens, inadequate or irregular drug supply, poor compliance by patients, malabsorption of one or more drugs, and sequestered disease (in which differential penetration of anti-TB drugs may lead to monotherapy) have been reported as reasons for developing drug resistant strains.7,8
Progressive clinical and/or radiographic deterioration or failure of cultures to convert in a timely fashion while the patient is receiving treatment should lead to the anticipation of treatment failure, and possible acquired drug resistance.9 Among these patients, depending upon the circumstances, consideration should be given to changing the treatment regimen. If a decision is made accordingly, the essential principle is to add at least two new drugs, preferably three, to which the organism is known to be susceptible or those that the patient has never received and never introduce just a single drug to a failing regimen.9
The change-over from supervised sanatorium treatment to unsupervised domiciliary treatment has affected compliance significantly. In Portugal there are no explicit legal grounds on which to enforce TB's treatment on those that refuse it, and certain patients are extremely difficult to follow-up. The success of the treatment relies in a relationship based on mutual trust; therefore it is extremely important that the medical teams do not underestimate non-compliance determinants.
When compliance is difficult to predict, the most effective method of drug delivery is directly observed therapy (DOT) rather than self-administered therapy. The provision of DOT should be prioritized to (1) suspected or proven drug-resistant organisms, (2) treatment failure, (3) documented re-treatment disease, (4) drug abuse/homelessness, (5) suspected or previous non-compliance, (6) mental illness, (7) sputum smear positive for acid-fast bacteria, (8) HIV co-infection, and (9) children.7–9
Although affected by a selection bias, the HIV co-infection did not appear to be a predisposing factor for the development of MDR-TB (Table 2). Global surveys suggest that HIV is not an independent risk factor for the development of drug resistance.5 Many MDR-TB nosocomial outbreaks have been reported among HIV patients.7,10 However, if patients with HIV infection are treated appropriately, it is not evident that it favors the transmission of MDR strains,7,10 although there might be shared risk factors between them (drug injection, homelessness, hospitalization and others).7
In Coimbra District there has been an increase of the proportion of cases resistant to at least one first line anti-TB drug, a typical feature of low incidence regions. The most powerful predictor of the presence of MDR-TB is a history of previous treatment. The implications of our findings relating to RMP support the restriction of its use to protect its efficacy.1–5
The high proportion of MDR-TB cases resistant to PZA highlights an important alert, because it can become an important public health problem, as PZA is an essential part of TB treatment, widely used for both drug-susceptible and MDR-TB treatment. Thus, its DST is critical, particularly among MDR-TB patients, before starting or adjusting treatment regimens.4
Overall the study findings emphasize the importance of continuing to prioritize the prevention of drug-resistant TB strains from developing through: (1) continuous epidemiological surveillance in order to monitor drug resistance patterns; (2) prescription of appropriate treatment; and (3) assurance that the prescribed regimen is complied with, and that those who abscond from treatment are identified early and duly supervised under DOT.
We should all keep in mind that the potential to induce resistances as result of insufficient compliance will never be completely overcome, even with the development of new drugs.
Conflict of interestThe authors have no conflicts of interest to declare.
We would like to extend our gratitude to Manuel Veloso dos Reis (M.D.), to Carla Nunes (Ph.D.) and António Jorge (M.D., Ph.D.) for their valuable insight and opinions.
Equally, we would like to thank the professionals of the health facilities; that in some way allocated some of their valuable time to collaborate with the study.