Red blood cell distribution width (RDW) is associated with increased mortality risk in patients with chronic obstructive pulmonary disease (COPD). However, limited data are available for critically ill patients with COPD.
MethodsData from the Medical Information Mart for Intensive Care III V1.4 database were analyzed in this retrospective cohort research. The International Classification of Diseases codes were used to identify critically ill patients with COPD. The first value of RDW was extracted within the first 24 h after intensive care unit admission. The endpoint was 28-day all-cause mortality. Multivariable logistic regression analysis was performed to examine the relationship between RDW and 28-day mortality. Age, sex, ethnicity, anemia status, comorbidities, clinical therapy, and disease severity score were considered for subgroup analysis.
ResultsA total of 2,344 patients were included with mean (standard deviation) age of 72.3 (11.3) years, in which 1,739 (53.6%) patients were men. The increase in RDW was correlated with an increased risk of 28-day mortality in the multivariate logistic regression model (odds ratio [OR] 1.15; 95% confidence interval [CI] 1.09−1.21). In comparison with the low-RDW group, the middle and high-RDW groups tended to have higher risks of 28-day all-cause mortality (OR [95% CI] 1.03 [0.78−1.34]; OR [95% CI] 1.70 [1.29−2.22]; P trend < 0.0001). Subgroup analyses show no evidence of effect modifications on the correlation of RDW and 28-day all-cause mortality.
ConclusionAn increase in RDW was associated with an increased risk of 28-day all-cause mortality in critically ill patients with COPD. Further studies are required to investigate this association.
COPD is an irreversible, progressive airway inflammatory disease characterized by chronic respiratory symptoms and airflow limitation.1 According to the Burden of Obstructive Lung Diseases and other large-scale epidemiological studies, patients with COPD increased to 384 million in 2010, with a global prevalence of 11.7% (95% confidence interval [CI] 8.4%–15.0%) in 2010 and 12.16% (95% CI 10.91–13.40%) in 2015.2,3 Across the World Health Organization regions, the USA have recorded the highest prevalence of COPD (13.3% in 1990, 15.2% in 2010, and 14.53% in 2015).2,3 Furthermore, COPD is the third leading cause of death globally according to the Global Burden of Disease.4 Thus, COPD is a substantial clinical and financial burden with significant influence on patients’ quality of life and healthcare expenditure. Red blood cell distribution width (RDW) is a quantitative measure of circulating erythrocyte volume variability, and it is measured as part of a complete blood count examination. Furthermore, RDW has significant associations with the risk of adverse clinical outcomes in patients with coronary artery disease,5-17 heart failure,18,19 stroke,20,21 acute pulmonary embolism,22-25 community-acquired pneumonia,26-28 peripheral occlusive artery disease,29 cancer,30,31 sepsis,32 and kidney disease.33-35 Furthermore, an increase in RDW is associated with right ventricular dysfunction,36,37 pulmonary arterial hypertension,37 and mortality.38-41
However, previous studies employed small sample sizes and did not adjust for other potential confounders. In addition, limited studies have focused on the correlation between RDW and outcome in critically ill COPD patients.
Thus, the present study aimed to investigate the association between RDW and 28-day all-cause mortality in critically ill COPD patients by using the Medical Information Mart for Intensive Care III (MIMIC-III) database. We hypothesized that increasing RDW in critically ill COPD patients is related to increased risk of mortality.
MethodsDatabase introductionMIMIC-III database (version 1.4) is open to the public and contains information on over 50,000 patients hospitalized to the Beth Israel Deaconess Medical Center's intensive care unit (ICU) between 2001 and 2012.42 This database can be accessed at https://mimic.physionet.org/. Enqian Liu obtained access to the database (No. 35919439). The use of the data was approved by the Beth Israel Deaconess Medical Center (Boston, MA) and Institutional Review Boards of the Massachusetts Institute of Technology (Cambridge, MA).43 The requirement for written informed consent was waived, because each patient information in the database was anonymized and de-identified.43 The ethics committee of Lishui Municipal Central Hospital approved this study (no. 2021184).
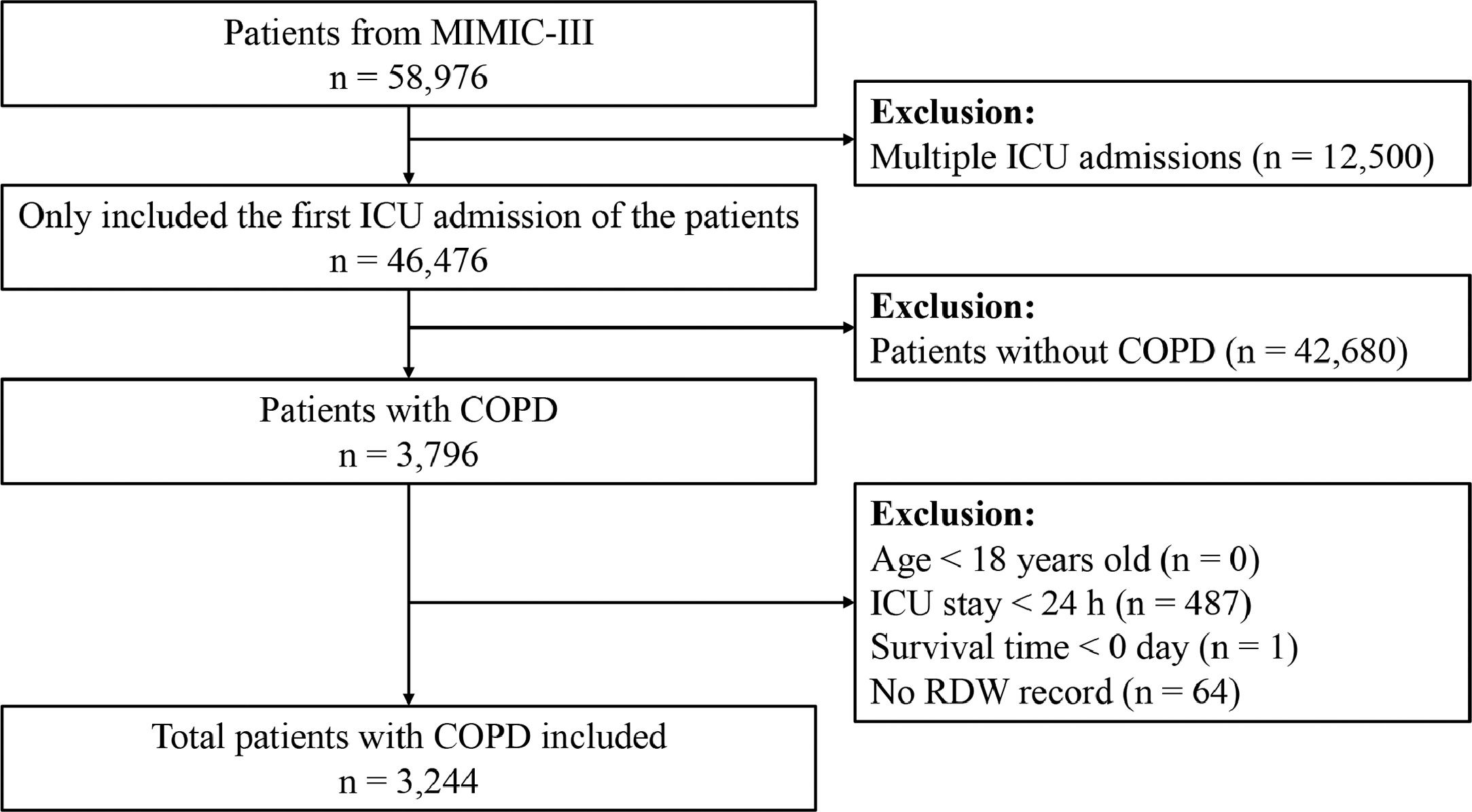
Population selection criteriaData from each patient's initial ICU admission were analyzed. Patients with COPD were selected using International Classification of Diseases, Ninth Revision (ICD-9) codes (491.20, 491.21, 491.22, and 496). We excluded patients aged <18 years, those admitted to the ICU for <24 h, those with survival times <0, and those with missing RDW data.
Data extractionData were extracted using PostgreSQL (version 9.6) and a structured query language. The following variable data were extracted: age, sex, ethnicity, weight, heart rate, systolic blood pressure (SBP), diastolic blood pressure (DBP), temperature, respiratory rate, percutaneous oxygen saturation (SpO2), mean blood pressure (MBP), vasopressor use, renal replacement therapy (RRT), congestive heart failure, hypertension, diabetes, cardiac arrhythmias, sepsis, liver disease, acute kidney injury (AKI), renal failure, serum sodium, serum creatinine, serum potassium, blood urea nitrogen (BUN), serum hemoglobin, serum bicarbonate, serum glucose, serum hematocrit, anion gap, serum chloride, platelet, and white blood cell (WBC), Sequential Organ Failure Assessment (SOFA) score, and Simplified Acute Physiology Score II (SAPS II). The baseline data were obtained within the first 24 h after ICU admission. The initial value was considered for a variable that was measured multiple times within 24 h after ICU admission. Comorbidities, except AKI, were diagnosed according to ICD-9 codes. Severe sepsis was defined as the presence of either (a) a combination of ICD-9 codes for infection and one or more organ dysfunctions,44 or (b) the ICD-9 code for severe sepsis (995.92) or septic shock (785.52).45 According to the Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guidelines, AKI was induced within the first 24 h of ICU admission and was identified based on serum creatinine and urine output.46 Anemia was diagnosed as a hemoglobin level <12 g/dL in women and <13 g/dL in men according to the World Health Organization guidelines.47 The clinical outcome was the 28-day all-cause mortality after ICU admission.
Statistical analysisParticipants were divided into tertiles based on RDW levels. Continuous data were expressed as mean (standard deviation) or median (interquartile range), while categorical variables were presented as percentages. The baseline characteristics of the different RDW tertile groups were analyzed using chi-square test for categorical variables, one-way analysis of variance test for normally distributed data, and Kruskal-Wallis H test for non-normally distributed data. The relationship between RDW and 28-day all-cause mortality was determined using multivariate logistic regression analysis. Values of the variation inflation factor (VIF) were used to assess multicollinearity. More than 10 VIFs showed multicollinearity. We constructed three models, namely, model 1 that was unadjusted, model 2 that was adjusted for age, sex, and ethnicity, and model 3 that was adjusted for age, sex, ethnicity, and other variables with P < 0.1 in univariate analysis or those with >10% change in effect estimates (weight, SBP, MBP, heart rate, respiratory rate, SpO2, hematocrit level, platelet level, anion gap, creatinine level, bicarbonate, chloride, glucose, BUN, WBC, and potassium levels, SAPS II, SOFA, cardiac arrhythmias, hypertension, sepsis, liver disease, vasopressor use, ventilation, RRT, AKI, and anemia). To investigate the non-linearity further, we turned RDW into a categorical variable based on the tertiles, and then into a continuous variable by entering the tertiles’ median values into the variable. For the sensitivity analysis, interaction and subgroup analyses were based on age (<75 and ≥75 years), sex, ethnicity, congestive heart failure, diabetes, renal failure, hypertension, cardiac arrhythmias, liver disease, sepsis, anemia, AKI, RRT, vasopressor use, ventilation, SAPS II (<37 and ≥37), and SOFA score (<4 and ≥4). Missing values were not found in the category variables. Missing values for continuous variables were imputed using the mean (normal data) or median (non-normal data). The statistical significance was considered at p < 0.05. Data analysis was performed using the R statistical software package (version 4.1.1).
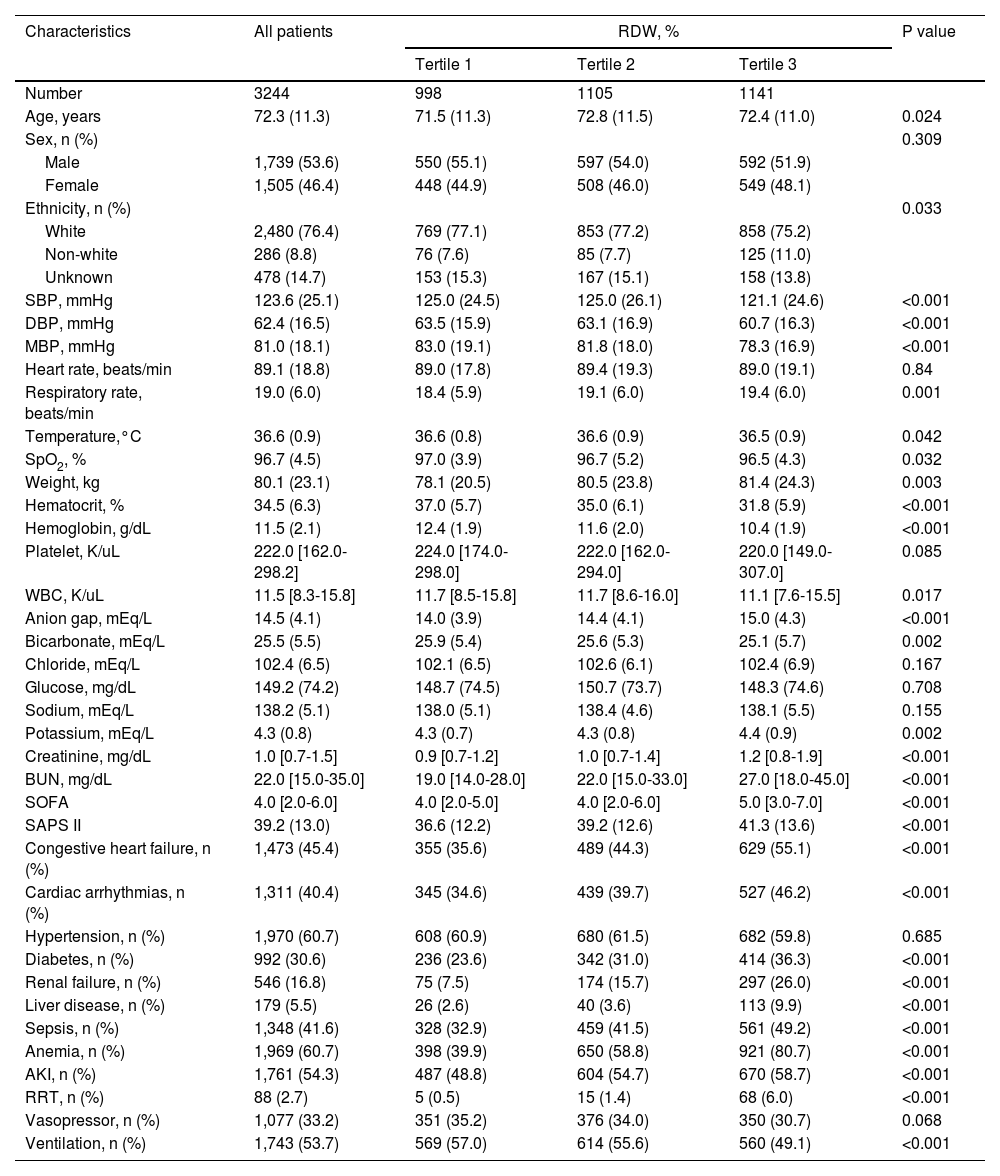
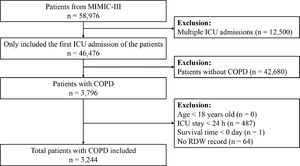
ResultsBaseline characteristics of the included participantsA total of 3,244 patients in the MIMIC-III database satisfied the inclusion criteria (Fig. 1). The baseline characteristics of the RDW tertile groupings are shown in Table 1. The mean patient age was 72.3 ± 11.3 years, and approximately 53.6% patients were men. RDW values at baseline varied from 11.50% to 28.20% (median 14.70%; mean 15.18%). No significant differences were observed between the different groups concerning sex, heart rate, platelet level, serum chloride, glucose, and serum sodium levels, hypertension, and vasopressor use (all P > 0.05). Patients in the highest RDW tertile group were likely to develop congestive heart failure, cardiac arrhythmias, diabetes, real failure, liver disease, sepsis, anemia, AKI, and RRT, and were less likely to require ventilation than patients in the lowest group. As the RDW increased, respiratory rate, weight, anion gap, potassium, creatinine, and BUN levels, SOFA score, and SAPS II increased, whereas SBP, DBP, MBP, temperature, SpO2, and hematocrit, hemoglobin, WBC, and bicarbonate levels decreased.
Patient characteristics according to tertiles of red blood cell distribution width.
Continuous variables are presented as means (SDs) or medians (quartiles), while categorical variables are presented as absolute numbers (percentages). MBP, mean blood pressure; BUN, blood urea nitrogen; WBC, white blood cell; SOFA, Sequential Organ Failure Assessment; SAPS II, Simplified Acute Physiology Score II; AKI, acute kidney injury; RRT, renal replacement therapy.
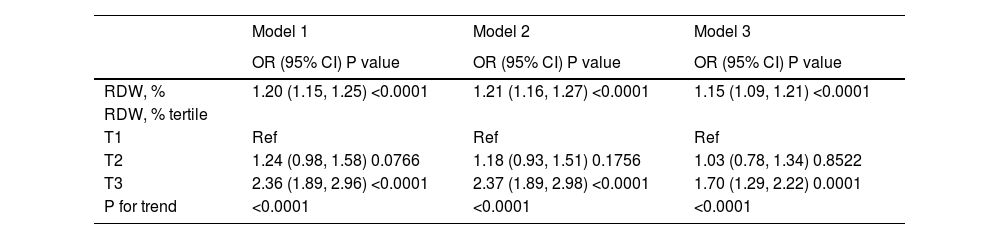
The independent effects of RDW on 28-day all-cause mortality in critically ill COPD patients were evaluated by constructing three different logistic regression models.
Logistic regression analysis for 28-day mortality (Table 2) show that RDW was positively related to the risk of 28-day all-cause mortality (unadjusted odds ratio [OR] 1.20; 95% CI 1.15−1.25). The crude ORs were 1.24 (95% CI 0.98−1.58) and 2.36 (95% CI 1.89−2.96) in the second and third tertile groups of RDW, respectively, with the first RDW tertile group as the reference. After adjusting for age, sex, and ethnicity, higher RDW values were correlated to higher risks of 28-day mortality (OR 1.21; 95% CI 1.16−1.27). In comparison with the first tertile group, the ORs were 1.18 (95% CI 0.93−1.51) and 2.37 (95% CI 1.89−2.98) in the second and third tertile groups, respectively. RDW was strongly correlated with 28-day all-cause mortality (OR 1.15; 95% CI 1.09−1.21) in Model 3. Furthermore, a higher RDW value was related to a greater risk of 28-day all-cause mortality in the second RDW tertile group (OR 1.03; 95% CI 0.78−1.34) and the third RDW tertile group (OR 1.70; 95% CI 1.29−2.22) after adjusting for age, sex, ethnicity, weight, SBP, MBP, heart rate, respiratory rate, SpO2, hematocrit level, platelet level, anion gap; creatinine, bicarbonate, chloride, glucose, BUN, WBC, and potassium levels, SAPS II, SOFA score, cardiac arrhythmias, hypertension, sepsis, liver disease, vasopressor use, ventilation, RRT, AKI, and anemia. The linear trend tests for 28-day mortality yielded remarkable results in the three different models.
Relationship between red blood cell distribution width and 28-day all-cause mortality in different models.
OR, odds ratio; CI, confidence interval; Ref, reference; RDW, red blood cell distribution width
Model 1 was not adjusted; Model 2 was adjusted for age, sex, and ethnicity; and Model 3 was adjusted for age, sex, ethnicity, weight, systolic blood pressure, mean blood pressure, heart rate, respiratory rate, percutaneous oxygen saturation, hematocrit level, platelet level, anion gap, creatinine, bicarbonate, chloride, glucose, blood urea nitrogen, white blood cell, potassium levels, Simplified Acute Physiology Score II, Sequential Organ Failure Assessment score, cardiac arrhythmias, hypertension, sepsis, liver disease, vasopressor use, ventilation, renal replacement therapy, acute kidney injury, and anemia.
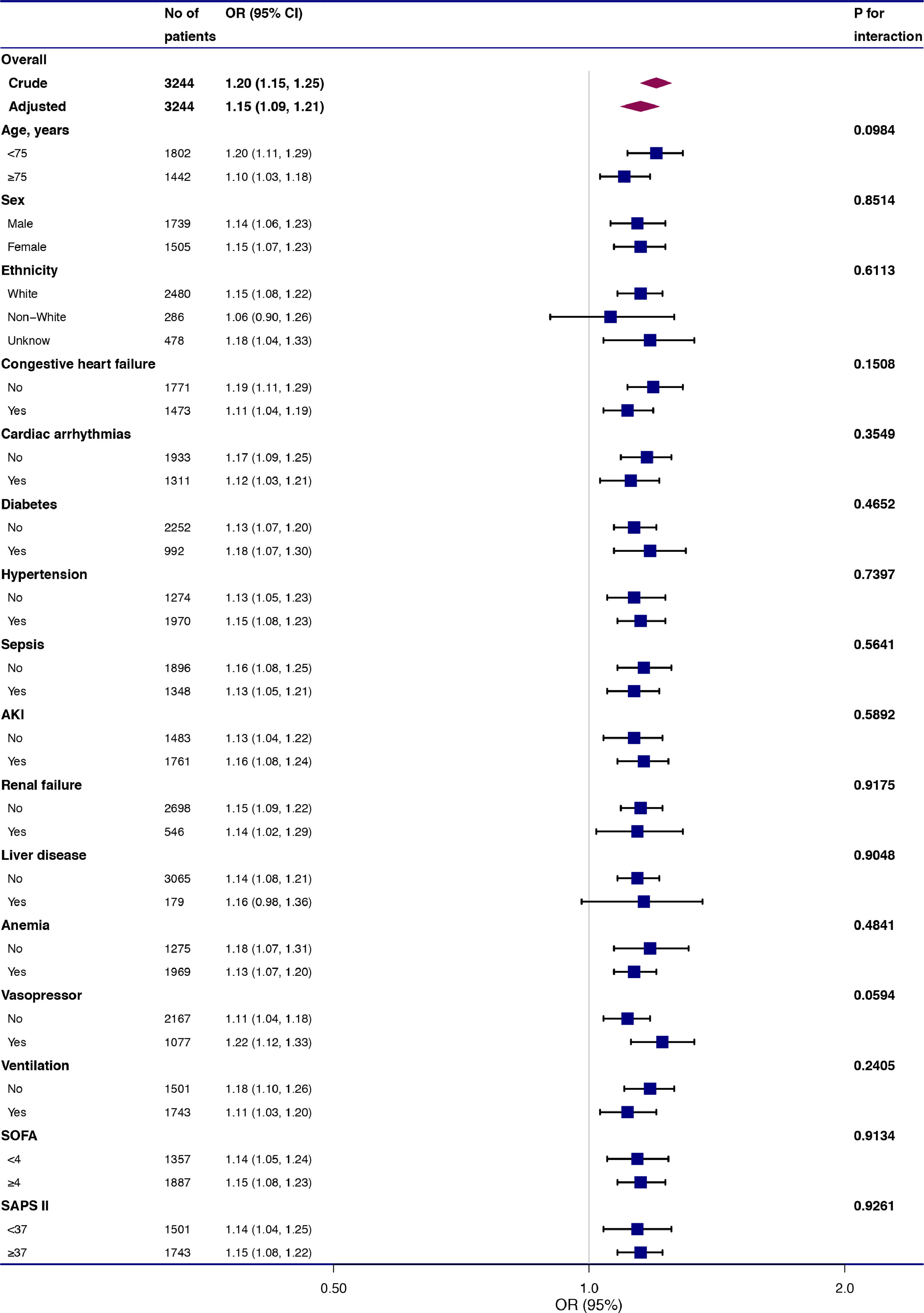
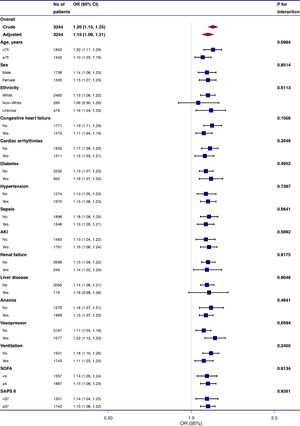
To evaluate the underlying clinical heterogeneity, we used interaction and stratified analyses (Fig. 2). We assessed the relationship between RDW and 28-day mortality in different subgroups. Interaction and stratified analyses were not detected in terms of age (<75 and ≥75 years), sex, ethnicity, anemia, diabetes, hypertension, cardiac arrhythmias, renal failure, liver disease, congestive heart failure, sepsis, anemia, AKI, RRT, vasopressor use, ventilation, SAPS II (<37 and ≥37), and SOFA score (<4 and ≥4).
Effect size of red blood cell distribution width on 28-day mortality in prespecified and exploratory subgroups. The effect size was adjusted for age, sex, ethnicity, weight, systolic blood pressure, mean blood pressure, heart rate, respiratory rate, percutaneous oxygen saturation, hematocrit level, platelet level, anion gap, serum creatinine, bicarbonate, chloride, glucose, blood urea nitrogen, white blood cell, and potassium levels, Simplified Acute Physiology Score II, Sequential Organ Failure Assessment score, cardiac arrhythmias, hypertension, sepsis, liver disease, vasopressor, ventilation, renal replacement therapy, acute kidney injury, and anemia, except for the subgroup variable.
In this retrospective cohort study, higher RDW values were independently related with increased risks of 28-day all-cause mortality in critically ill patients with COPD. In addition, the stratified result supports the consistent finding.
RDW is related to short- and long-term mortality in patients with internal diseases. In recent years, RDW has received substantial attention in patients with COPD. Seyhan et al.38 retrospectively followed up 270 patients with stable COPD for a median period of 36 months (range, 20−52 months) and found that higher RDW levels are related to higher mortality risks (OR 1.12; 95% CI 1.01−1.24). After controlling for age, leukocyte count, mean corpuscular volume, thrombocytopenia, and anemia, results show that RDW was related to increased risk of in-hospital mortality in a study among 330 patients with acute COPD exacerbations.39 Epstein et al.40 discovered that high RDW at admission (>14.5%) was significantly related to the 60-day composite endpoint of readmission or mortality after discharge (OR 1.83; 95% CI 1.22–2.74) in a cohort of 539 patients with acute exacerbations of COPD. Hu et al.41 conducted a prospective observational research among 442 patients with acute exacerbations of COPD and observed that increased RDW (≥13.75%) was strongly correlated with the risk of in-hospital death (relative risk 4.30; 95% CI 1.98−9.58) and the risk of 1-year mortality (HR 1.64; 95% CI 1.08−2.50). However, previous research involved limited sample sizes, and adjustments were not made for a large number of potentially confounding factors. Furthermore, to the best of our knowledge, limited research has focused on the correlation between RDW and mortality in critically ill patients with COPD. Our results support these earlier investigations.38-41 In the present study, which included 3,244 critically ill patients with COPD, an increase in RDW was related to the increased risk of 28-day all-cause mortality based on multivariate logistic regression analysis.
Aging48-51 and ethnicity52,53 are associated with RDW values. Different epidemiological studies have reported inconsistent results in terms of the relationship between RDW and sex.49-51 The correlation between RDW and 28-day mortality was constant across all subgroups in our analysis, regardless of age (<75 and ≥75 years), sex, or ethnicity. COPD comorbidities include hypertension, congestive heart failure, diabetes, and cardiac arrhythmias, and comorbidities mainly cause mortality among COPD patients.54 Sepsis and AKI frequent occur in ICU patients and are related with poor outcomes.55,56 The results of RDW and mortality were stable in these subgroups. RDW is commonly used for anemia differential diagnosis. Higher RDW levels are related to an increased risk of death, regardless of presence or absence of anemia. The effects of treatment and illness severity score should be considered. The results were consistent in different subgroups according to vasopressor use, ventilation, SAPS II (<37 and ≥37), and SOFA score (<4 and ≥4).
The mechanisms underlying the increase in RDW are unclear. To the best of our knowledge, high RDW level is correlated with an inflammatory state. Inflammation can affect iron metabolism and bone marrow function, thus inhibiting erythropoietin-induced erythrocyte maturation.57,58 This condition results in the release of immature red blood cells into the circulation and disruption in red blood cell clearance, thus increasing RDW. Commonly, critically ill patients exhibit systemic inflammatory responses.59 These mechanisms may assist in exploring the correlation between RDW and poor outcomes in COPD patients. Thus, RDW may be related to poor outcomes in patients with COPD.
Our study has some limitations. First, considering that the present study involves observational research, causal inferences cannot be detirmined. Moreover, the analysis was adjusted for the available confounders, but our observations may have been influenced by residual measured and/or unmeasured confounders. In addition, we excluded patients aged <18 years. Therefore, our findings cannot be generalized to these patients. Furthermore, RDW values could have been affected by many factors, such as erythropoietin use and iron or vitamin B12 deficiency, although considering the retrospective nature of the study, these situations could not be distinguished. Moreover, we only focused on the initial RDW value obtained within the first 24 h after ICU admission. The effect of RDW fluctuations on prognosis is unknown. In addition, the ICD-9 code-based definition of severe sepsis may underestimate the actual incidence of sepsis. Some cases of sepsis may not be covered by the codes. Finally, considering that this study was a single-center, retrospective database analysis, our findings must be validated using a multicenter, prospective survey with a larger sample size.
ConclusionsThis cohort study suggests that an increase in RDW is associated with a higher risk of 28-day all-cause mortality in critically ill patients with COPD.
Data availabilityThe data used in the present study may be obtained by sending an email to the author (jiangwx90@163.com). However, approval should be obtained from the MIMIC III Institute to re-analyze the complete data.
ContributionLEQ was in charge of the study design and data collection. LWH analyzed data and contributed to writing this paper. SDB, LWW, ZJS, ZJH, and JM all joined in the discussion and examined the article. JWX designed and supervised the study. Finally, all writers approved the final manuscript.