Malignant pleural mesothelioma (MPM) is a locally aggressive and invasive tumour with a median survival of 12 months.1 For the past few decades, it has also been considered a highly lethal condition because of its recurrence despite standard approaches. Currently, there is no recommended therapy for relapsed MPM after chemotherapy or front-line treatment and disease control has been less than 30% in all previous studies of second-line drugs.2 Advances in immunotherapy have been shifting the paradigm in the treatment of several advanced cancers and, more recently, its value in MPM has been investigated as a possible future therapeutic option.2,3
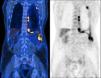
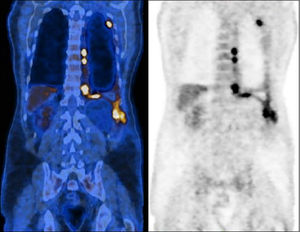
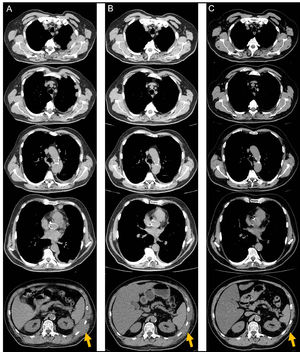
A sixty-two-year-old male, active smoker, with type-2 diabetes mellitus, arterial hypertension, and a history of prolonged occupational exposure to asbestos in the past had an incidental finding of unilateral complex and exudative pleural effusion associated with massive thickening of the costodiaphragmatic pleura. Uniportal video-assisted thoracic surgery with pleural decortication was performed to manage complex pleural effusion and to collect samples that secured the pathological diagnosis. Post-surgery study was complemented by positron emission tomography scanning (Fig. 1), clarifying the diagnosis of an unresectable epithelioid MPM, with appearance of chest wall invasion. The patient was initially submitted to pemetrexed (500 mg/m2) and carboplatin (area under the concentration-time curve: 5), a less nephrotoxic regimen considering his comorbidities, which was suspended after six treatment cycles due to disease progression, deterioration in Eastern Cooperative Oncology Group (ECOG) performance status to 2 and development of left posterolateral thoracic mass, painful on palpation, needing antalgic radiotherapy (total dose of 30 Gy in 10 fractions over 2 weeks). Chest computed tomography (CT) scan also reported dimensional lesion increase and clear invasion of the chest wall (Fig. 2A). In this context, anti-programmed cell death-1 (PD-1) monoclonal antibody immunotherapy was administered with single agent intravenous nivolumab 3 mg/Kg, every two weeks. Initial outpatient reassessments were performed on a 14-day schedule. It was possible to witness a progressive improvement in symptoms and weight recovery; after seven weeks the chest-CT showed near complete remission of the neoplastic lesion (Fig. 2B). The thoracic painful mass became non-palpable at the end of the fourth nivolumab cycle. Adverse events monitoring was performed before each treatment infusion. He presented moderate arthralgia as a possible immune-related side-effect, which was controlled with daily 5 mg prednisolone, without interrupting therapy. At the time this report was written, the patient has been maintained on 3 mg/Kg nivolumab infusions every two weeks, with an excellent sustained therapeutic response (Fig. 2C) and significant improvement in quality of life (current ECOG 1), without unacceptable pharmacological toxicity, maintaining benefits that came from immunotherapy throughout subsequent evaluations and well more than 24 months after diagnosis.
Positron emission tomography scan prior chemotherapy, demonstrating a dispersed hypermetabolic thickening through the left pleura, especially the costodiaphragmatic region, invading the chest wall, and an homolateral pleural effusion with heterogeneous uptake of F-18 fluorodeoxyglucose (SUVmax 10.6).
Serial reassessments by computed tomography scan documenting the lesions’ extension and evolution. (A) After 6 treatment cycles of carboplatin-pemetrexed chemotherapy, reflecting recurrence of disease in the left hemithorax. (B) After 4 treatment cycles of nivolumab, demonstrating parcial but impressive response. (C) After 37 treatment cycles of nivolumab with no evidence of recurrence and maintaining remarkable tumour response.
In fact, epithelioid tumours account for 60% of mesothelioma subtypes and have the best prognosis with more favourable response to chemotherapy than the other forms.1,4 However, this report demonstrates the progressive character of malignant mesothelioma despite standard chemotherapy and less aggressive histologic type. Patient's declining status, low response to second-line therapies and chemotherapy-related complications contributed towards the use of immunotherapy as an off-label rescue strategy. This decision was also based on preliminary results from recent clinical trials that have suggested a potential role of anti-PD-1 monoclonal antibody in relapsed MPM, namely nivolumab as a single drug, with an acceptable safety profile, which was important to improve our patient compliance and tolerance.2,3,5 Positive outcomes were rapidly achieved and confirmed from the first control-CT, maintaining a near-complete and sustained response ever since, largely exceeding expected survival time for this malignancy, with significant improvement in quality of life. PD-ligand1 (PD-L1) expression has been reported in 40% of mesothelioma overall, with a higher rate in sarcomatoid histotype.6 Nevertheless, this case is representative of epithelioid subtype and, although PD-L1 tumour proportion score has not been investigated, the impressive obtained response with nivolumab might support a dependency of mesothelioma on this immunological checkpoint.7 As described in the literature, tumour immune microenvironment plays a key role in MPM pathogenesis, but to date, efficacy of PD-L1 expression status as a predictive biomarker for the response to nivolumab may be limited;7 this is a critical area for more extensive studies. Lastly, immune checkpoint inhibitors are not without side-effects but, in this case, the potential benefit with nivolumab monotherapy outweighed the reported manageable adverse events.
We here present a case of epithelioid MPM which experienced a rapid disease progression after initial therapy but then had an exceptional and sustained response to single agent nivolumab. It is an impressive shift in prognosis by a novel rescue strategy, exceeding expected survival time and quality of life for this malignancy. Therefore, it highlights a promising role for this anti-PD-1 monoclonal antibody in future therapeutic options in those patients who have progressed after pemetrexed–platinum doublet chemotherapy. This example should strongly encourage research for biomarkers to select optimal candidates for immunotherapy in terms of efficacy and tolerance.
Patient consentWritten informed consent was obtained from the patient for publication of his clinical details and images.
FundingNo funding source.
We would like to acknowledge Luís Castelo, MD, radiologist, for his collaboration on the review and selection of CT images, and Maria José Lima for English revision.