To investigate the respiratory function and lung hyperinflation in asymptomatic smokers without previous pulmonary pathology and with normal chest radiography. To identify tobacco-related diseases and to correlate tobacco consumption, duration of exposure to tobacco smoke and urinary cotinine with the existence of tobacco-related disease.
Material and methodsCase-controlled study with pairing by sex, age, and body mass index. Case definition: smokers who presented to the first appointment of smoking cessation at the Hospital Sousa Martins (HSM) without respiratory symptoms and with normal chest radiography. Definition of control: users without current and/or past tobacco exposition and with plethysmography and chest radiography at HSM within normal parameters.
ResultsReductions in FEV1/FVC, FEF 75%, FEF 25–75% and the cardiothoracic index were detected in smokers and showed a moderated inverse correlation of TLC (with statistical meaning) compared with the control group. Approximately 31.2% of the smokers showed extrapulmonary disease related to tobacco, and 9.38% of the smokers exhibited subclinical chronic obstructive pulmonary disease (COPD). Smokers with tobacco-related diseases presented a mean age and RV/TLC ratio superior to smokers without pathology.
DiscussionThe reduction of the mean values of FEV1/FVC, FEF 75%, FEF 25–75% and the cardiothoracic index seems to indicate precocious pulmonary dysfunction. This work aims to reveal the importance of detecting premonitory anomalies of pulmonary disease during the subclinical phase in patients at risk. Smoking must be considered a factor of multisystemic repercussion; thus, intervention opportunities in this particular group must not be wasted. This preliminary study identifies potentially promising variables with the aim of testing the hypothesis that there can be premonitory alterations in COPD, according to its evolution versus reversibility after smoking cessation. This work will be concluded in a future study.
Smoking has devastating health effects and is a recognized risk factor for the development of cardiovascular, cerebrovascular, respiratory, and oncological diseases.1 The Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommends the diagnosis of chronic obstructive pulmonary disease (COPD) based on a reduction in the FEV1/FVC ratio.2 However, this diagnostic criterion is valid only for advanced COPD, leading to the underdiagnosis of early functional changes in chronic smoking.3 There are few studies concerning pulmonary function alterations in smokers and the deleterious effects of smoking on functional parameters vary, depending on the studies.
Cigarette smoking has a detrimental effect on the airways, causing inflammation and, consequently, airflow limitations and lung hyperinflation.4,5 In thoracic radiographs, one sign of lung hyperinflation is the reduction in the cardiothoracic index (‘heart in gout’). Some authors claim that a reduction in FEV1 is more than just a measure of airflow limitation, that it is, in fact, a marker of premature death, with a wideranging utility in assessing the risks of COPD, lung cancer, coronary artery disease, and stroke, which collectively account for 70–80% of premature deaths in smokers.6 Some studies have encouraged the use of new methods in the detection of precursory anomalies of pulmonary disease during the subclinical phase in patients at risk.
The authors present a case control study developed in outpatient clinic with the smoking cessation program at Sousa Martins Hospital (HSM), Local Health Unit, Guarda (Portugal). The main objective of our study was to investigate the respiratory function and lung hyperinflation in asymptomatic smokers without a history of lung pathology and with normal chest radiographs. Secondary objectives consisted of identifying tobacco-related diseases and correlating tobacco consumption, duration of exposure to tobacco smoke, and urinary cotinine with the existence of tobacco-related disease.
Materials and methodsThe authors developed a case-controlled study paired by sex, age and body mass index. The cases were defined as smokers (with a smoking habit for at least one year), who presented for their first appointment at the smoking cessation program at HSM during the period of 11 December 2012 to 8 December 2013, without respiratory symptoms and with normal chest radiographs. Smoking subgroups were integrated using evidence of the history of tobacco-related pathology. The exclusion criterion was applied to cases previously diagnosed with pulmonary pathology.
The controls were participants who exhibited normal examination in plethysmography and chest radiographs in HSM. The following exclusion criteria were applied to the controls: known prior history of pulmonary pathology and current and/or past smoking. In the controls, maximum passive exposure was assumed for the determination of carbon monoxide (CO) in the exhaled air (0–6ppm) and urinary cotinine (<150ng/mL).
The pulmonary function tests were performed using the Plethysmograph Autobox DI SensorMedics 6200 (California, USA) at the Department of Pathophysiology of Respiratory Diseases at HSM. The technical recommendations and standardized reference values of the American Thoracic Society were used to perform the functional tests. A chest X-ray was obtained while the patient was in the standing and forward positions at maximal inspiration, which complies with the criteria for good technical quality. The ratio between the maximum transverse diameter of the heart and the maximum internal diameter of the chest (the calculation of the cardiothoracic index) was determined. A normal thoracic radiograph was considered to be one without pleuroparenchymal alterations and with a cardiothoracic index below 0.5.
As a biomarker of current smoking, a determination of the CO in the exhaled air was used. Cotinine in the urine was used as a marker for the cumulative consumption of tobacco intake. The measurement of the CO in the exhaled air was performed during the consultation period of smoking cessation in the Micro Medical smoke check (Kent, England). Assays were stratified into the following CO levels: 0–6ppm, green=no smoking; 7–10ppm, yellow=light smoker; 11–20ppm, red=strong smoker; and >20ppm, red with beep=very strong smoker. The assay of urinary cotinine was executed using gas/mass spectrophotometry chromatography. The assays of urinary cotinine were stratified as follows: <150ng/mL=passive smoking; 150–499ng/mL=light smoker; 500–2500ng/mL=moderate smoker; and >2500ng/mL=strong smoker.
The criteria for hyperinflation included the presence of either a reduction in the cardiothoracic index or an increase in RV/TLC measured using plethysmography. The minimum value of the cardiothoracic index that defines hyperinflation is not yet clearly established in the literature.
Statistical analyses were performed using IBM SPSS Statistics software 19®. Exploratory and descriptive analyses were applied to the variables, and in the continuous variables, such as the T-test (2 groups) and the Pearson correlation coefficient. The level of significance was set at p<0.05.
ResultsDuring the study period, 64 subject samples were obtained: 32 cases and 32 controls. The 32 cases included smokers in the smoking cessation program at HSM: 65.6% were male, and 34.4% were female. The mean age of male subjects was 45.57 years, and that of female subjects was 37.27 years. There were statistically significant differences between the groups (p<0.05). The controls included 32 non-smoking individuals, matched with cases by sex, age, and body mass index. The lung function tests were performed at the Division of Respiratory Pathophysiology, HSM. Tables 1 and 2 characterize the cases by gender in terms of age at onset of smoking, tobacco consumption, urinary cotinine assay, and CO in exhaled air.
Characteristics of smokers by gender.
| Variables | Smokers (n=32) | |||
|---|---|---|---|---|
| Male subjects(n=21)Mean±standard deviation | Female subjects(n=11)Mean±standard deviation | Total(n=32)Mean±standard deviation | T test(p value) | |
| Body mass index (kg/m2) | 26.45±4.17 | 25.06±5.02 | 25.97±4.45 | 0.41 |
| Age (years) | 45.57±11.63 | 37.27±6.74 | 42.72±10.86 | 0.038 |
| Age of smoking initiation (years) | 16.67±4.73 | 16.83±3.66 | 16.72±4.33 | 0.927 |
| Tobacco consumption (packs per year) | 30.33±14.46 | 12.73±5.26 | 24.28±14.69 | 2.91×10−5 |
| Urinary cotinine (ng/mL) | 1315.33±895.45 | 699.91±465.00 | 1103.78±821.74 | 0.042 |
Determination of CO in exhaled air in smokers by gender.
| Determination of CO in exhaled air (ppm) | Smokers (n=32) | ||
|---|---|---|---|
| Male subjects(n=21)n (%) | Female subjects(n=11)n (%) | Total(n=32)n (%) | |
| 0–6=‘non-smoker’ | 0 (0%) | 0 (0%) | 0 (0%) |
| 7–10=‘light smoker’ | 0 (0%) | 1 (9.09%) | 1 (3.13%) |
| 11–20=‘strong smoker’ | 10 (47.62%) | 8 (72.73%) | 18 (56.25% |
| >20=‘very strong smoker’ | 11 (52.38%) | 2 (18.18%) | 13 (40.63%) |
It was found that the age of onset of smoking was similar in both genders. Female smokers had a mean tobacco consumption (12.73 packs per year) and mean cotinine (699.91ng/mL), which was lower than that of the male subjects (30.33 packs per year and 1315.33ng/mL), with statistical significance (p<0.05). With regard to CO in exhaled air, 84.62% of the male subjects were considered to be strong smokers vs. 15.38% of the female subjects. The mean duration of exposure to smoking in male subjects was 28.9 years and 20.45 years for female subjects, with statistically significant differences (p<0.05).
There was a weak correlation between urinary cotinine and tobacco consumption (r=0.124, p=0.5).
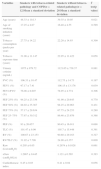
A review of the cases and controls using plethysmography is shown in Table 3. In the cases identified, 9.38% (n=3) of the smokers exhibited spirometric COPD criteria: mean FEV1/FVC 65% and mean FEV1 89%. The mean FEV1/FVC of the cases was 77.78% (standard deviation [SD]=6.50%), with 81.41% in the controls (SD=5.84%), p<0.05. The means of the FEF 75% and FEF 25–75% were also lower. The mean FEF 75% was 63.78% (SD=26.44%) in the cases and 86.09% in the controls (SD=28.99%), p<0.05. The mean FEF 25–75% in the cases was 83.16% (SD=26.44%) and 97.19% (SD=23.82) in the controls (p<0.05). The reduction was greater in the FEF 75% and FEF 25–75% parameters.
Evaluation by plethysmography of smokers and controls.
| Respiratory function tests | Smokers(n=32)Mean±standard deviation | Controls(n=32)Mean±standard deviation | T test(p value) |
|---|---|---|---|
| FVC (%) | 110.34±13.49 | 108.84±14.87 | 0.674 |
| FEV1 (%) | 102.78±12.06 | 106.13±12.96 | 0.289 |
| FEV1/FVC (%) | 77.88±6.50 | 81.41±5.84 | 0.026 |
| FEF 25% (%) | 92.78±21.47 | 88.91±17.44 | 0.431 |
| FEF 50% (%) | 94.75±31.86 | 105.34±26.61 | 0.154 |
| FEF 75% (%) | 63.78±26.44 | 86.09±28.99 | 0.002 |
| FEF 25–75% (%) | 83.16±26.44 | 97.19±23.82 | 0.029 |
| PEF (%) | 91.41±17.61 | 87.59±16.79 | 0.379 |
| TLC (%) | 104.09±13.42 | 100.16±12.79 | 0.234 |
| RV (%) | 94.5±27.11 | 88.97±19.88 | 0.356 |
| RV/TLC (%) | 27.53±7.63 | 27.81±6.00 | 0.870 |
| Airway resistance – raw (L/s/cmH2O) | 0.2440±0.091 | 0.2615±0.120 | 0.513 |
| Specific airway conductance – sGaw (cmH2O/L/s) | ♂1.122±0.564 | ♂1.1057±0.477 | ♂0.920 |
| ♀1.300±0.4306 | ♀1.543±0.640 | ♀0.309 |
The reductions in FEV1/FVC, FEF 75% and FEF 25–75% were correlated inversely with tobacco consumption, but this was not statistically significant (r=−0.305 and p=0.089, r=−0.203 and p=0.265, r=−0.15 and p=0.391, respectively).
Regarding the assessment of the cardiothoracic index, the mean was 0.424 (SD=0.04) in the cases and 0.458 (SD=0.04) in the controls, with a statistically significant difference (p<0.05). A moderate correlation between the cardiothoracic index and TLC was found in the cases (r=−0.371, p<0.05).
Approximately 31.2% (10) of the cases had a history of extra-pulmonary pathology associated with tobacco: 21.88% (seven patients) had hypertension, 6.25% (two cases) had stroke, 3.13% (one case) had acute myocardial infarction and 3.13% (one case) had repeated miscarriages. 9.38% (three smokers) were diagnosed with COPD. In 68.8% (22 smokers), there was no history of tobacco-related disease. Table 4 shows the comparison of the cases in subgroups, with and without tobacco-related disease, in relation to age, age at smoking onset, tobacco consumption, duration of tobacco exposure, urinary cotinine assay, plethysmography variables, and cardiothoracic index. In relation to the distribution by gender in these two subgroups, it was verified that among the smokers with tobacco-related pathology (n=12), eight were male and six were female; among smokers without tobacco-related pathology (n=20), 13 were male and seven were female.
Analysis of subgroups of smokers with and without tobacco-related pathology.
| Variables | Smokers with tobacco-related pathology and COPD(n=12)Mean±standard deviation | Smokers without tobacco-related pathology(n=20)Mean±standard deviation | T test(p value) |
|---|---|---|---|
| Age (years) | 48.33±10.13 | 39.35±10.05 | 0.021 |
| Age of smoking initiation (years) | 17.25±4.07 | 16.40±4.55 | 0.599 |
| Tobacco consumption (packs per year) | 27.75±14.22 | 22.20±14.93 | 0.309 |
| Tobacco exposure time (years) | 31.08±11.147 | 22.95±11.422 | 0.058 |
| Urinary cotinine (ng/mL) | 1075±979.72 | 1121.05±738.37 | 0.881 |
| FVC (%) | 106.33±10.47 | 112.75±14.73 | 0.197 |
| FEV1 (%) | 97.17±7.41 | 106.15±13.176 | 0.039 |
| FEV1/FVC (%) | 76.08±8.607 | 78.95±4.774 | 0.306 |
| FEF 25% (%) | 94.25±23.336 | 91.90±20.843 | 0.770 |
| FEF 50% (%) | 88.92±35.367 | 98.25±29.963 | 0.431 |
| FEF 75% (%) | 57.17±29.634 | 67.75±24.253 | 0.280 |
| FEF 25–75% (%) | 77.67±30.312 | 86.46±23.676 | 0.368 |
| PEF (%) | 93±20.657 | 90.45±16.011 | 0.698 |
| TLC (%) | 101.47±9.09 | 105.7±15.448 | 0.391 |
| RV (%) | 100.67±23.153 | 90.80±29.163 | 0.327 |
| RV/TLC (%) | 31.92±7.798 | 24.90±6.357 | 0.009 |
| Raw (L/s/cmH2O) | 0.205±0.05 | 0.2674±0.1028 | 0.061 |
| sGaw (cmH2O/L/s) | 1.2867±0.445 | 1.121±0.565 | 0.393 |
| Cardiothoracic index | 0.45±0.03 | 0.41±0.04 | 0.056 |
It was found that the mean age of the patients with tobacco-related pathologies was 48.33 years (SD=10.13 years), which was higher than that of the group without pathologies (39.35 years, SD=10.05 years), with statistically significant differences between the two groups (p<0.05). The mean duration of exposure to tobacco was higher in the subgroup with tobacco-related pathologies (31.08 years, SD=11.12), compared with the subgroup without pathologies (22.95 years, SD=11.42), although without statistical significance.
With regard to the RV/TLC ratio, the cases with tobacco-related diseases had a higher mean (31.92%, SD=7.80%) compared with the subgroup without pathologies (24.90%, SD=6.36%, p<0.05). The cases with the tobacco-related pathologies (12 cases) had higher tobacco consumption and lower cotinine compared with the subgroup without pathologies but without statistically significant differences (p>0.05).
DiscussionThis study reflects a sample of asymptomatic smokers from the respiratory point of view, with high tobacco consumption. The age of onset of smoking was similar in both genders. The female subjects who presented to the HSM smoking cessation program were younger than the male subjects (mean age lower, p<0.05). Tobacco consumption, mean duration of exposure to tobacco smoke, and urinary cotinine levels of the female subjects were below in comparison with of the male subjects (p<0.05).
There were reduced FEV1/FVC, FEF 75%, FEF 25–75%, and cardiothoracic indices in cases and moderate inverse correlations between the TLC and cardiothoracic index, with statistical significance. The potential value of these variables was validated using the statistical significance found in the reduced sample. At the time of our study, 31.2% of the smokers showed extra-pulmonary pathologies related to tobacco consumption. Three smokers (9.38%) were diagnosed with subclinical COPD. Smokers with tobacco-related diseases had a higher mean age, a lower FEV1, and greater RV/TLC compared with smokers without pathologies (p<0.05). These findings show individual susceptibility to the development of pulmonary disease in later life and/or show a decline of pulmonary function by age; this phenomenon was identified by Flecher. There was no statistical significance in terms of smoking history, mean duration of exposure to tobacco smoke, and urinary cotinine. This is determined by the homogeneity of the groups regarding tobacco consumption, because all the included smokers presented with high tobacco consumption.
As indicated in the previous studies, the FEV1 and FEV1/FVC ratio are not sensitive markers of early changes in lung pathophysiology or the emergence of COPD in smokers. Structural and functional changes are present in the smoker's lungs before the onset of clinical signs of airway obstruction. Thus, the underdiagnosis of early functional changes in chronic smoking represents an opportunity for early intervention.3,7
Our sample reflects a population of smokers (young adults without underlying lung pathologies) with declining respiratory function parameters (reduction of mean values of FEV1/FVC, FEF 75% and FEF 25–75%) and lung hyperinflation compared with adult non-smokers in the same age group. In smokers with a history of tobacco-related extra-pulmonary disease, these changes may indicate pulmonary dysfunction at an early stage. We need to know which subgroup of individual susceptibility factors confer greater propensity for the harmful effects of tobacco; that is, which subgroup indicates that systemic disease with multiple organ involvement in COPD occurs as a late manifestation of the disease? The changes found in our study may reflect the premonitory signs of the progression to COPD at a later stage of disease in patients with extra-pulmonary pathologies related to tobacco.
In conclusion, smoking must be considered a disease which has potentially multisystemic repercussions. The study of the subgroup of patients with previous extrapulmonary pathologies related to tobacco has particular relevance for the demonstration of a greater individual susceptibility to the development of pulmonary alterations in a later spectrum of the illness. These findings show that a reduction of the mean values of FEV1/FVC, FEF 75%, FEF 25–75% and the cardiothoracic index may be indicative variables for the precocious detection of these patients.
This study has some limitations, in particular, the small sample size, the inclusion of a lower number of female smokers than male smokers, and the absence of evaluation of diffusing capacity for CO (DLCO).
With this preliminary study, we have sought to increase awareness of the variables that should be included in a prospective study; we intend to conduct future comparisons in the respiratory function variables and the cardiothoracic index of smokers before and after smoking cessation. These studies will aim to assess the possible reversibility of the changes found after smoking cessation.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors of this study would like to thank all of their colleagues and the technicians at the Sousa Martins Hospital, Local Health Unit, Guarda who participated in this study.