Adaptive servoventilation is a recent ventilatory mode initially designed to treat Cheyne–Stokes respiration (CSR). Recently, the efficacy of ASV has been discussed for the treatment of central sleep apnea (CSA) and treatment-emergent central sleep apnea (treatment-emergent CSA) where other forms of traditional positive airway pressure (PAP) may be insufficient.
ObjectivesTo compare the clinical impact of ASV with other forms of PAP in treating patients with treatment-emergent CSA, CSA and CSR.
MethodsMedical data of all the patients who underwent polysomnography (PSG) with ASV titration were evaluated. The patients were divided into two groups according to the mode of ventilation reimbursed: ASV and PAP (AutoCPAP/CPAP/BIPAP). All patients had a minimal follow-up of 6 months. Both groups were compared in terms of symptoms, apnea hypopnea index, compliance, cardiac function and cardiovascular events.
ResultsASV titration was performed in 33 patients (30M/3F) with a mean age of 69±8 years. The majority (58%) present a treatment-emergent SA and 42% a CSA and or CSR. The median initial diagnostic AHI was 46±22events/h.
After the initial diagnosis, 28 patients were treated with PAP and 5 with servoventilation. All of the patients treated with PAP were posteriorly submitted to PSG and ASV titration because of suboptimal response to PAP. Despite a clear indication for ASV, due to differences in reimbursement, 15 patients continued treatment with PAP (12 with AutoCPAP, 1 with BIPAP and 2 with CPAP) and 16 changed to ASV. Two patients were lost in follow-up.
In both groups, most of patients present a treatment-emergent SA (53% in ASV group vs. 67% in PAP group) or a CSA/CSR (29.4% in ASV group vs. 20% in PAP). After ASV titration, the mean follow-up was 25±14 months. Both groups (ASV vs. PAP) were similar in terms of compliance (77±23% vs.88±14%) and in terms of Epworth sleepiness scale score (6±5 vs. 7±5). There was a statistical difference in terms of residual AHI: mean AHI was 4±3 in ASV group and 9±3 in PAP group (P=0.005). We found no differences in terms of left ventricular fractional shortening (ASV 33±10% vs. PAP 32±10%). Although no difference was observed between the 2 groups in terms of non-fatal cardiovascular events (3 events in each group), 2 fatal cardiovascular events occurred in the PAP group (sudden death).
ConclusionsThese data confirm that ASV is an efficient treatment in patients with treatment-emergent CSA, CSA/CSR significantly decreasing residual AHI. In both groups, compliance rate was high and sleepiness improved. It is relevant that the 2 patients who died of sudden death were treated with PAP.
Central sleep apnea is characterized by a lack of drive during sleep resulting in insufficient or absent ventilation and compromised gas exchange. The lack of respiratory efforts during cessation of airflow may lead to frequent nighttime awakenings, with consequent excessive daytime sleepiness and increased risk of adverse cardiovascular outcomes.1
Cheyne–Stokes respiration (CSR) is a disorder characterized by recurrent central apneas during sleep alternating with a crescendo-decrescendo pattern of tidal volume. It is observed in patients with congestive heart failure, usually during stages 1 and 2 non-REM sleep when ventilation is under chemical-metabolic control.2,3
In recent years, sleep physicians have recognized that some patients with obstructive sleep apnea develop central apneas or CSR after initial treatment with positive airway therapy. That sleep disorder is called treatment-emergent central sleep apnea. The significance and the prevalence of treatment-emergent SA is not clear; it ranges from 2.5% to 20%.4–10 There is some controversy around the optimal treatment of CSA syndrome and treatment-emergent central sleep apnea.
Adaptive servoventilation is a recent ventilatory mode, able to provide a dynamic adjustment of inspiratory pressure support. ASV continuously calculates a target minute ventilation, breath-by-breath, increasing or decreasing the pressure support in order to avoid transient episodes of central hypopnea/apnea after hyperventilation and associated hypocapnia.
The first commercial ASV devices became available in 2006. The device used for PSG in this study is AutoSet CS®. The device provides a fixed end-expiratory pressure adjusted to treat obstructive events and a respiratory frequency back-up rate. The inspiratory pressure is adjusted by the device in order to obtain a calculated target ventilation (90% of the patient's recent average ventilation) of a running 3min reference period.
The present study is a retrospective case-series comparison of the efficacy of traditional non-invasive positive pressure ventilation (PAP) and ASV in patients with diagnosis of treatment-emergent central sleep apnea, central sleep apnea and or Cheyne–Stokes respiration.
MethodsPatientsWe identified all the patients referred to ASV titration at our Sleep Medicine Center. Most of the patients had previously undergone an unsuccessful PAP trial.
DefinitionsThe ASV titration was done by split-night PSG (SomnoStar® Sleep System). Sleep stage scoring was performed according to standard criteria of the American Association Sleep Medicine (2007). An apnea was defined as ≥90% airflow reduction for ≥10s, and a hypopnea was defined as ≥30% reduction in airflow for ≥10s accompanied by ≥3% desaturation from baseline. An arousal was defined as ≥3-s increase in EEG frequency following ≥10s of stable sleep, accompanied by an increase in submentalis EMG activity for ≥1s during REM sleep. The AHI was calculated as the number of apneas and hypopneas per hour of sleep. The arousal index was calculated as the number of arousals per hour of sleep. Obstructive sleep apnea syndrome was diagnosed if RDI was ≥5 events and the patient was symptomatic (daytime sleepiness, nocturnal gasping or choking, loud snoring with description of breathing interruptions) or if RDI was >15 even if patient was asymptomatic. CSA was diagnosed if the number of central apnea per hour was ≥5 and at least 50% of the total AHI was central in origin. Treatment-emergent SA was diagnosed if CPAP titration eliminated obstructive events but the residual central apnea index (CAI) was ≥5 or the CSR pattern became predominant.
Study designAfter the baseline sleep study, patients diagnosed with treatment-emergent CSA, CSA or CSR were submitted to a second PSG (SomnoStar® Sleep System) for ASV titration. Titration was performed online by the neurophysiologist technician in sleep laboratory. Medical charts were reviewed and demographic, clinical and polygraphic/polysomnographic data were extracted.
After PSG with ASV titration, the eligible subjects were allocated into 2 groups: patients who received flow-triggered ASV (BiPAP Auto SV Phillips and VPAP Adapt SV Resmed) and those who received PAP (AutoCPAP/CPAP/BIPAP) (REMStar Auto Philips and Auto Spirit S8/S9). Both groups had a minimal follow-up of 6 months. The selection of the ventilator mode was exclusively based on differences in reimbursement.
In the CPAP group, airway pressure was manually modulated from 6cmH2O to the effective pressure, with a maximum of 14cmH2O. The appropriate fixed pressure was chosen as the pressure abolishing or significantly decreasing both obstructive events (including apnea, hypopnea, and snoring) and CSR without arousal.
After a minimum time of 6 months, residual IAH, compliance, Epworth sleepiness scale, left ventricular shortening fraction and cardiovascular events were recorded. Residual AHI was given by the ventilator support device data.
Statistical methodsStatistical analysis was performed using SPSS version 17.0 for Windows. All values are shown as the means SD, and categorical variables are expressed as numbers and percentages. The baseline characteristics were compared using Student's t test for continuous variables, whereas the Chi-square test or Fisher's exact test was used for categorical variables. Within- and between-group comparisons of measurements were carried out using paired and unpaired Student's t tests for normally distributed data, and the Wilcoxon-signed rank test for non-normally distributed data.
Values <0.05 were considered statistically significant.
ResultsDemographics and clinical findingsThirty-three patients were included: 30 men and 3 women with an average age of 68±8 years.
Patient baseline characteristics before any positive pressure therapy are summarized in Table 1. Of the 33 patients submitted to ASV titration, 16 were allocated to ASV, 15 to the PAP and 2 patients were lost in follow-up. In PAP group, 12 patients were treated with AutoCPAP, 1 with BIPAP and 2 with CPAP.
Patient baseline characteristics before any positive pressure therapy.
| ASV group (n=16) | PAP group (n=15) | P value | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (yr) | 71±6 | 66±10 | 0.11 |
| Body mass index (kg/m2) | 30±4 | 31±5 | 0.39 |
| Heart failure, n (%) | 4 (29) | 2 (13) | 0.67 |
| Hypertension, n (%) | 9 (59) | 7 (47) | 1 |
| Dysrhythmia, n (%) | 4 (23) | 4 (27) | 0.13 |
| Diabetes mellitus, n (%) | 5 (29) | 4 (27) | 1 |
| Stroke, n (%) | 0 (0) | 1 (7) | 0.39 |
| Sleep respiratory variables of initial diagnosis | |||
| AHI (events/h) | 44±18 | 52±25 | 0.34 |
| Central apnea index (/h) | 10±12 | 4±6 | 0.16 |
| Obstructive apnea index (/h) | 18±23 | 17±16 | 0.91 |
| Mixed apnea index (/h) | 4±4 | 9±15 | 0.27 |
| Hypopnea index (/h) | 16±10 | 13±10 | 0.45 |
| Cheyne–Stokes, n (%) | 6 (37) | 6 (40) | 0.55 |
| Mean nocturnal SaO2 (%) | 92±4 | 92±4 | 0.94 |
| Minimal nocturnal SaO2 (%) | 72±14 | 76±12 | 0.44 |
| Epworth sleepiness scale | 12±7 | 11±6 | 0.78 |
| Arterial blood gases | |||
| PaCo2 (mmHg) | 38±3 | 37±7 | 0.59 |
| PaO2 (mmHg) | 89±13 | 91±3 | 0.56 |
| HCO3 (mmol/L) | 25±3 | 25±3 | 0.97 |
| ASV group (n=9) | PAP group (n=7) | P value | |
|---|---|---|---|
| Hemodynamic parameters | |||
| LVFS (%) | 35±15 | 35±4 | 0.96 |
Heart failure was present in 20.6% of cases, hypertension in 53% and dysrhythmia in 12%. Twenty-nine percent of the patients had history of diabetes mellitus.
In both groups (PAP vs. ASV) there were no statistical differences in terms of age (mean 71 years±6 vs. 66±10; P=0.11), sex (88% male vs. 93%), BMI (mean 30±4 vs. 31±5; P=0.39), Epworth sleepiness scale (mean 12±7 vs. 11±6; P=0.78) or diagnostic AHI (mean 44±18 vs. 52±25). There was also no significant difference in terms of cardiovascular comorbidities (Table 1).
As this study is retrospective, not all the patients had an initial echocardiogram available, prior to PSG with ASV titration; in fact, echocardiographic evaluation before and after ASV titration was only available in 16 patients (9 in ASV group vs. 7 in PAP group). The median of the initial left ventricular shortening fraction was 35±15 in ASV group and 35±4 in PAP group with no differences between them (P=0.96).
The diagnosis that led to an ASV study was treatment-emergent SA in 59.4% (44.4% in ASV group vs. 55.6% in PAP group), CSA and/or CSR in 40.6% (in 61.5% ASV group vs. 38.5% in PAP).
The indication for ASV titration was a suboptimal response to PAP. The summary of polysomnographic features of split-night of ASV titration is summarized in Table 2. During polysomnography ASV titration, the mean AHI was 7±10events/h after optimal pressure for ASV titration and the mean cumulative percentage time at a pulse oximetry oxygen saturation <90% was 11±27%.
Polysomnography findings of ASV titration.
| PSG ASV titration | |
|---|---|
| Apnea hypopnea index (events/h) | 7±10 |
| Central apnea index (/h) | 1±4 |
| Obstructive apnea index (/h) | 4±7 |
| Mixed apnea index (/h) | 2±8 |
| Respiratory events related arousals (/h) | 1±3 |
| Respiratory arousal/awakening index | 20±16 |
| Mean O2 sat | 94±2 |
| Min O2 sat | 89±4 |
| SpO2<90% (%) | 11±27 |
| Sleep efficiency | 69±17 |
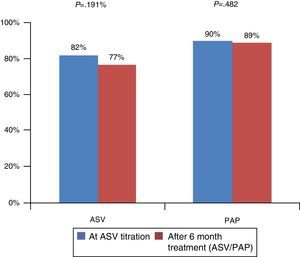
After ASV titration, the mean follow-up was 25±14 months. Both groups (ASV vs. PAP) were considered compliant as they used their device for more than 4h per night and were similar: 77±23% in ASV group (P=0.191) and 88±14% in PAP group (P=0.482) (Fig. 1) (P=0.153). The average number of hours of daily use was 6h23±2h13 in ASV group and 6h59±1h08 in PAP group.
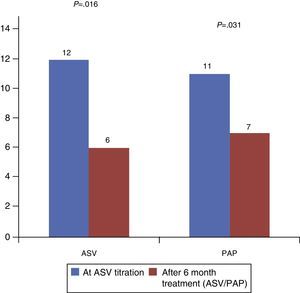
In terms of sleepiness, both groups improved the Epworth sleepiness scale score after the initial diagnosis with a statistically significant difference (P=0.016 in ASV group vs. P=0.031 in PAP group). However, comparing only the final Epworth, no significant statistical difference was found between them: 6±5 in ASV group and 7±5 in PAP group (P=5.06) (Fig. 2).
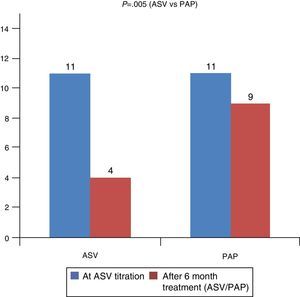
As shown in Fig. 3 there was a statistical difference in terms of residual AHI: mean AHI was 4±3 in ASV group and 9±3 in PAP group (P=0.005).
We found no differences in terms of blood gases or left ventricular fractional shortening (ASV 33±10% vs. PAP 32±10%).
Table 3 includes a summary of cardiovascular events. There was no difference in terms of non-fatal cardiovascular events (3 events in each group) but in PAP group 2 patients died of sudden death. These 2 patients had treatment-emergent SA and the ventilator mode was CPAP in one patient and BIPAP in the other. Both of them had diabetes, hypertension and alcoholism. One suffered from heart failure.
DiscussionThe efficacy of ASV for the treatment of Cheyne–Stokes respiration (CSR) associated with cardiac heart failure (CHF) was first reported in 2001 and confirmed subsequently in several short term studies.11–13 Since then, longer-term studies (3–6 months) have compared ASV to CPAP and indicated that ASV is more efficient than CPAP in improving LVEF and decreasing the AHI over baseline.14–16
Concerning other causes of central sleep apnea a recent study analyzed the role of ASV in treating patients with neurological or idiopathic central sleep apnea. This long-term study had a mean follow-up of 36±18 months and concluded that ASV improved their AHI from 47.4±19.8 to 6.9±9.3/h [P<0.001] and mean nocturnal SaO2 from 92.1±2.6% to 93.6±3.2% [P<0.001].17
Regarding treatment of treatment-emergent CSA, there is an important debate. Some studies argue that central sleep apnea events disappear a few months later with the maintenance of CPAP therapy6–8 but our experience has taught us that some central apneas persist even with the regular CPAP therapy. Several studies have analyzed the role of ASV in the treatment of treatment-emergent SA and have concluded that ASV is a leading treatment.18 In the largest case series to date, ¾ of the 63 patients with Treatment emergent SA exhibited a drop in AHI to <10/h on ASV concluding that ASV is also an excellent option for these patients.19
In our study, we demonstrate that ASV titration is an efficient treatment for patients with treatment-emergent CSA, CSA or CSR as it shows significant decreases of AHI and on Epworth sleepiness scale, compliance rate was high (with 77% compliant patients in ASV group) and the long-term tolerance was excellent.
Compared to PAP, results for our sample fully agree with previous studies since we found a statistical difference between the 2 groups in terms of AHI.
We did not demonstrate a difference between ASV and PAP in terms of LVFS improvement but only 16 patients had cardiac function evaluation before and after PSG for ASV titration and treatment decision. Therefore, it is difficult to evaluate the effect of the 2 types of ventilation concerning cardiac function because of the small number of patients.
We also did not find differences in terms of non-fatal cardiovascular events between the 2 groups but in the PAP group 2 patients died of sudden death.
There are some limitations of this study. First of all, some of the patients, because of problems due to reimbursement, were not treated with ASV although they had indication to start that mode of ventilation mode, so the number of the patients in the ASV group was small. Furthermore, as the study is retrospective, not all the patients had previously had an echocardiogram before PAP therapy so the comparison between the two ventilator modes concerning cardiac function cannot be made with confidence because of the small number of patients who had echocardiogram before and after PAP therapy.
ConclusionASV is an effective treatment in patients with treatment-emergent CSA, CSA/CSR significantly decreasing residual AHI. In both groups, compliance rate was high and sleepiness improved. It is clinically relevant that the 2 patients who died of sudden death were treated with PAP.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.