The role of flexible bronchoscopy (FB) in patients diagnosed with community-acquired pneumonia (CAP) is not clearly defined. The etiologic diagnosis of CAP is known to be a medical problem.1 In about 40% of CAP cases the causative organism remains unknown.2 The most common microorganisms in CAP are Streptococcus pneumoniae (S. pneumoniae), although recent studies have described an increased frequency of other microorganisms. Proper treatment of CAP is crucial and early FB may change the prognosis especially in non-resolving CAP, FB can play a key role in the clinical outcome.1
Investigate the contribution of FB in patients with non-resolving CAP in relation to etiological diagnosis, change in treatment strategy, days of hospitalization and hospital mortality.
A retrospective analysis of patients with non-resolving CAP admitted to the Pulmonology ward of Coimbra University Hospital submitted to FB during the year 2014 was carried out. Information on clinical, radiological, laboratory, specifically microbiology aspirates and bronchial lavage and antibiotic treatment was collected. Non resolving CAP was defined as a persistence of pulmonary infiltrates in chest X-ray associated with persistence of typical respiratory symptoms in the absence of an alternative diagnosis despite 72h of antibiotic therapy.3 Bronchial aspiration was performed on all patients and when possible a bronchoalveolar lavage using 10–50ml of warm saline fluid was also performed. All specimens collected were sent to the microbiology laboratory. The criteria for infection of bronchial aspirates were the presence of colony forming units (CFU) greater than 105 per ml and bronchial lavage exceeding 104 CFU per ml. Intensive Care Unit patients and immunocompromised patients (very poor general condition, taking immunosuppressive drugs, with human immunodeficiency virus infection or active tuberculosis) were excluded from our study.
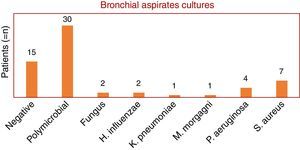
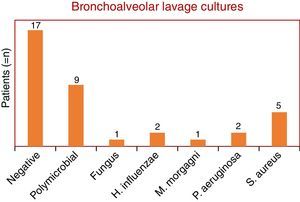
This study included 48 patients, 36 men and 12 women, with a mean and standard deviation age of 69±17 years vs 67±18, respectively. Non-resolving CAP accounted for 40% of all CAP admissions. Bronchial aspirate cultures were negative in 15 patients (24%), polymicrobial in 30 patients (49%) and positive in 17 patients (27%). Fungi were found in two patients (1 with Aspergillus fumigatus and 1 with Candida albicans). The bacterial microorganisms identified were: Haemophilus influenzae (two patients), Klebsiella pneumoniae (one patient), Morganella morganii (one patient), Pseudomonas aeruginosa (four patients) and Staphylococcus aureus (six methicillin-resistant (MRSA) and one methicillin-sensitive (MSSA)) (Graphic 1). Bronchoalveolar lavage was performed on only 37 patients, of whom 11 had a microorganism isolated as follows: C. albicans (one patient), H. influenzae (two patients), M. morganii (one patient), P. aeruginosa (two patients) and S. aureus (four MRSA and one MSSA). The remaining bronchial washings were negative in 22 patients and polymicrobial in 9 patients (Graphic 2). Empiric antibiotic therapy with penicillin group was initiated in half of the patients, quinolones in 15 patients, cephalosporins in seven, carbapenem in one and sulfonamides in another one. In 27% of cases (13 patients) antibiotic treatment was changed. Patients with no specific microorganism were hospitalized for an average of 13.6 days and patients with a specimen isolated for 11 days. In terms of mortality, two patients died: one with H. influenzae isolated and the other infected with MRSA. In terms of co-morbidities, hypertension was found in 45% of the patients, type 2 diabetes in 21%, dyslipidemia in 15% and acute renal failure in 6%. The majority (85%) had at least one co-morbidity associated.
The microorganisms identified in bronchoalveolar lavage coincided with the results in their bronchial aspirate. Surprisingly S. pneumoniae was not identified in any of the bronchial samples perhaps because of the urine antigen tests performed when the patients were admitted.4 The authors also highlight the high rate of MRSA in these immunocompetent patients which can be explained by several factors such as, age of the patients (mean 80 years), the presence of co-morbidities, including the worsening of renal function as demonstrated by Prina et al.5 and the use of non-selective antibiotic treatment which leads to the appearance of resistant bacteria.6
Simultaneously “atypical” microorganisms or viruses were not identified, which is unlike what Lim et al.7 reported. This result is due to early empirical antibiotics that decrease sensitivity of microbiological testing, the absence of an epidemic/pandemic outbreak during this study and urinary antigen of Legionella pneumophila search on admission.
All patients received treatment according to the Guidelines.3 It should be noted that the use of broad-spectrum antibiotic treatment took into account co-morbidities and previous antibiotic therapy and all patients whose antibiotic treatment was changed according to the microbiology results showed overall improvement.
Those patients in whom no specific microorganism was found were hospitalized for longer than patients with an isolated specimen. The identification of the microorganism responsible for the CAP with consequent alteration of the antibiotics may have influenced this result. Despite this trend there were no statistically significant differences between these two groups (p>0.05).
Only two patients died, both of them had multiple co-morbidities. The low mortality rate can be explained by early initiation of antibiotics and younger patients with fewer co-morbidities.
This study revealed that FB had limited value in patients with difficult to resolve CAP. Patients for whom FB led to a change of antibiotic therapy had fewer days of hospitalization and a good clinical outcome. Further studies addressing this issue need to be carried out.
Conflicts of interestThe authors have no conflicts of interest.