Organizing pneumonia (OP) is a histologic pattern of the lungs’ response to a wide variety of insults, including infectious, non-infectious, or no apparent reason (cryptogenic).1,2 It is usually a great mimicker showing a wide variety of signs, symptoms, and high-resolution computed tomography (HRCT) findings, which makes it a frequent differential diagnosis. The usual distribution in the HRCT is patchy, peribronchiolar with the presence of numerous buds of granulation tissue within alveoli, often involving alveolar ducts and small airways; areas of consolidation are also the characteristics of organizing pneumonia.2 Anatomopathological study is needed for final diagnosis.1,3,4 Although duration and initial symptoms depend on the underlying aetiology, OP usually presents with a several-month history of non-productive cough, low-grade fever, malaise, and shortness of breath. It is mostly seen in patients with pulmonary infection, drug reactions, transplantation, collagen vascular disease, granulomatosis with polyangiitis, after toxic-fume inhalation, e-cigarettes usage and, rarely, lung cancer of unknown primary site.1,5,6 When the underlying cause is not found, it is idiopathic and then called cryptogenic. Among the pulmonary infections, OP could be present after a bacterial, fungal, mycobacterial or even viral infection. Response to therapy is dependent on the treatment of the underlying cause but, usually, there is a good response to corticosteroid therapy with a good prognosis.3,4
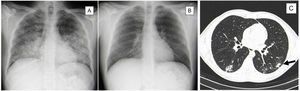
We report a case of a 35-year-old male, smoker of 15 packs per year, who went to the emergency department with pruriginous cutaneous eruption. Physical examination revealed vesicular cutaneous lesions, and on pulmonary auscultation a reduction of vesicular murmur and crepitations on the left hemithorax. Analysis identified respiratory failure, thrombocytopenia, hepatic dysfunction, and elevation of inflammatory parameters (C-Reactive Protein = 9.73 mg/dL (Normal < 0.5 mg/dL) with normal procalcitonin). The chest radiograph revealed bilateral opacities with air bronchogram (Fig. 1A). After the identification of epidemiological context for varicella-zoster infection (son with chickenpox), patient was admitted to hospital stay with the diagnosis of pneumonia due to Varicella-Zoster virus and medicated with intravenous acyclovir and levofloxacin for 7 days. A good clinical, analytical, and imaging response was observed (Fig. 1B). During the hospitalization, a thoracic computed tomography (CT) scan was done, showing multiple small nodules scattered in the pulmonary parenchyma, some forming small conglomerates and surrounding ground glass. They presented bilateral distribution, but predominantly in the lower lobes. The largest conglomerate measured was 15 mm in diameter and was located in the lower-left lobe. (Fig. 1C). The patient was discharged asymptomatic.
Imaging evolution of Varicella-Zoster pneumonia.
Posteroanterior chest radiograph evolution during Varicella-Zoster Pneumonia. A) On admission with bilateral opacities with air bronchogram. B) On discharge day showing almost complete resolution of the previously described lesions.
Thoracic computed tomography. C) On final days of hospitalization by varicella-zoster pneumonia showing multiple small nodules scattered in the pulmonary parenchyma, some forming small conglomerates, and surrounding ground glass with bilateral distribution, but predominantly in the lower lobes. The largest conglomerate measuring 15 mm in the lower left lobe (arrow).
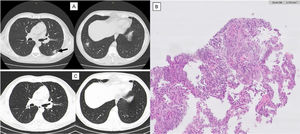
After one year, the patient returned to the emergency department with fever, odynophagia, cough, and haemoptysis, showing no changes in physical examination or blood analysis. The following thoracic CT revealed a micronodular pattern with mostly calcified nodules. In the lower right lobe, a dense 19 mm nodule with ground-glass pattern and, juxtaposed to this, other calcified 19 mm and 5.8 mm nodules were found. There was a growth of the previously reported lesion in the lower-left lobe, which measured 21 mm. (Fig. 2A).
Imaging evolution and histology of Organizing Pneumonia secondary to Varicella-Zoster infection.
Thoracic computed tomography (CT). A) One year after varicella-zoster infection showing a micronodular pattern with mostly calcified nodules. In the lower right lobe, a dense 19 mm nodule with ground-glass pattern and, juxtaposed to this, other two calcified nodules. Growth of the previously reported lesion in the lower-left lobe, measuring 21 mm (arrow). CT guided biopsy of the lower left lobe lesion. B) Anatomopathological exam showing focal lesions of organizing pneumonia with foamy macrophages in intralveolar localization. Thoracic CT. C) One year after corticosteroid treatment maintaining micronodular pattern with calcified nodules, but with complete resolution of the previously described lesions.
Blood and sputum culture were negative and bronchoalveolar lavage showed no changes. CT guided biopsy of the lower left lobe lesion was performed, with anatomopathological exam revealing focal lesions of organizing pneumonia with foamy macrophages in intralveolar localization (Fig. 2B). After the exclusion of other causes of organizing pneumonia and considering the previous diagnosis of varicella-zoster pneumonia, this cause was assumed. Corticosteroid therapy was started with an initial dose of oral prednisolone of 30 mg (0,5 mg/kg) daily for 6 months. Symptoms and imaging gradually improved and, after 1 year, clinical or imaging recurrence was not observed (Fig. 2C).
The present report describes a case of OP associated with varicella-zoster infection. Hypersensitivity pneumonitis (HP) could have a very similar clinical presentation such as fever, cough, and ground glass consolidations on CT and has a high incidence among Portuguese population in contact with birds, mould, cork or isocyanates.7 However, besides an identifiable exposure history, imaging changes are usually upper lobe predominant. Despite the usually good prognosis with response to antigen avoidance, some chronic forms, mainly fibrotic, could have the same prognosis as idiopathic pulmonary fibrosis.8 As previously stated, OP is an histological pattern associated with a variety of disorders,1,4 however, OP is rare after viral infections. There are a few cases reported of influenza association,5 while the association with varicella-zoster is even rarer. The few cases reported of OP after varicella-zoster infection occurred in patients with known risk factors for varicella-zoster infection, such as cigarette smoking, pregnancy, immunosuppression and male sex.9,10 Early diagnosis and treatment contribute to a favourable prognosis. We acknowledge the need for the physicians' awareness of the secondary OP after varicella-zoster infection, even if the disease had previously been cured.
Patient’s consentPatient’s informed consent was obtained.
Conflicts of interestThe authors have no conflicts of interest to declare.