Acquisition of Epidermal Growth Factor Receptor (EGFR) mutation resistance (mainly T790M) to EGFR tyrosine kinase inhibitors (TKI) occurs in about half of Non-Small Cell Lung Cancer (NSCLC) patients treated with TKI.1 Mutational status is important to guide therapy.2,3 Pirker stated that while tissue biopsy is currently the main source for molecular analyses, liquid biopsies will gain importance for diagnosis and disease monitoring in the future.4 The aim of this study was to analyse the value of liquid and tissue rebiopsy for evaluation of EGFR mutational status in a real-world setting.5,6
We carried out a retrospective identification of patients tested for EGFR mutation at a Portuguese cancer center between January 2015 and October 2019. We collected clinical data from patients with advanced EGFR mutation NSCLC. Descriptive statistic was used to analyse patients’ characteristics.
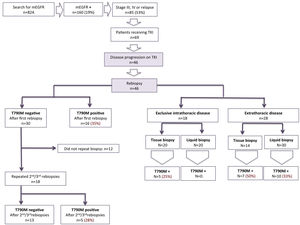
Of the 824 patients that were evaluated for EGFR mutation, 160 (19%) had EGFR-mutant NSCLC.
Eighty-five of 160 patients (53%) had advanced disease (11 patients with stage III, 65 patients with stage IV and 9 patients presented a relapsing NSCLC). All neoplasms were adenocarcinomas due to a selection bias of our institution (only adenocarcinmomas are tested for EGFR mutation. The median age at diagnosis was 69 years old [range: 39–95] and 66% of patients were female. History of tobacco use was reported as 42%.
The most common EGFR mutation was exon 19 deletion (del19) (33 patients) followed by L858R point mutation on exon 21 (24 patients) and del19 plus de novo T790M mutation (7 patients). Other uncommon mutations identified were L858R plus T790M, G719X on exon 18, del19 plus insertion of 20 and S7681 on exon 20 plus L858R.
Sixty-nine of 85 patients (81%) received EGFR-TKI and 46 patients (67%) developed disease progression on TKI. All 46 patients were submitted to a first (1st) rebiopsy, corresponding to a total of 50 rebiopsies: 25liquid biopsies and 25 tissue biopsies/cytologies (some patients had both). Eighteen patients underwent a second and third (2nd/3rd) rebiopsy, corresponding to a total of 34 rebiopsies: 25 liquid biopsies and 9 tissue biopsies/cytologies.
The most common sampling method was liquid biopsy both for 1st (50%) and 2nd/3rd rebiopsies (74%). Bronchial tissue was the most common site for tissue biopsy/cytology followed by pleural fluid both in 1st and 2nd/3rd rebiopsies. Less common sites were cerebrospinal fluid, thoracic lymph nodes and bronchial secretions for 1st rebiopsy and brain and bone for 2nd/3rd rebiopsies.
We analysed the proportion of T790M mutation identified for each rebiopsy site as shown in Table 1.
Rebiopsy sites and T90M detection success rate.
| 1st rebiopsy site | Number of biopsies | T790M + |
|---|---|---|
| Tissue biopsy/cytology | 25 | 11 (44%) |
| Bronchial tissue | 13 | 8 (62%) |
| Pleural fluid | 8 | 3 (38%) |
| Cerebrospinal fluid | 2 | 0 |
| Bronchial secretions | 1 | 0 |
| Thoracic Lymph node | 1 | 0 |
| Liquid biopsy | 25 | 6 (24%) |
| 2nd/3rd rebiopsy site | Number of biopsies | T790M+ |
| Tissue biopsy/cytology | 9 | 1 (11%) |
| Bronchial tissue | 4 | 0 |
| Pleural fluid | 3 | 0 |
| Brain | 1 | 1 (100%) |
| Bone | 1 | 0 |
| Liquid biopsy | 25 | 4 (16%) |
In 1st rebiopsy, bronchial tissue was the site where T790M mutation was most frequently identified (62%), followed by pleural fluid (38%) and liquid biopsies (24%). No T790M mutation was identified in other sites, corresponding to a T790M mutation detection rate of 44% for 1st tissue rebiopsy
In 2nd/3rd rebiopsy, T790M mutation was identified in the only brain biopsy performed and in 16% of the liquid biopsies. No T790M mutation was identified in other sites, meaning the detection rate for T90M mutation 2nd/3rd tissue rebiopsies was only 11%.
As shown in Fig. 1, 16 of 46 patients (35%) undergoing a 1st rebiopsy harbored a T790M mutation. Among the 30 remaining patients, 12 did not repeat biopsy and 18 were submitted to a 2nd/3rd biopsy. Five of those 18 patients (28%) were submitted to 2nd/3rd biopsies harbored a T790M mutation.
Furthermore, 18 of 46 patients undergoing rebiopsy presented exclusive intrathoracic disease. In this setting, T790M mutation was detected in 5 of 40 all rebiopsies (13%).
In patients with exclusive intrathoracic disease, liquid biopsy failed to identify T790M mutation, regardless of the number of rebiopsies. However, out of those 20 negative liquid biopsies, one had a positive matched tissue biopsy/cytology. T790M mutation was identified in 5 of 20 tissue rebiopsies/citologies performed (25%).
Among 28 patients with extrathoracic disease, T790M mutation was detected in 17 of 44 rebiopsies performed (39%). T790M mutation was found in 10 of 30 liquid biopsies (33%) and in 10 of 14 tissue biopsies/citologies (50%).
The overall survival was higher among patients submitted to rebiopsy [28.9 months (95%CI 21.2–35.0)] than among those were not [18.6 months (95%CI 9.87-NR)]. Although the increase of 10 months in overall survival was not statistically significant (p = 0.3116), this lack of significance might be related with the small number of our cohort. Additionally, we may consider that this tendency towards overall survival benefit could be related with a better performance status of the rebiopsied patients (fit to receive treatment). Also, we can hypothesize that rebiopsy might guide the physician to choose the best treatment, which would l lead to a better overall survival.
Our results suggest that 2nd/3rd biopsies are worth performing, as the proportion of patients with mutation identified is still significant and have a clinical impact in therapeutic choices and prognosis.7
T790M mutation is less identified in patients with exclusive intrathoracic disease. Liquid biopsy might not add value in this setting but tissue biopsy/cytology must be considered.
In extrathoracic disease, a higher proportion of T90M mutation was identified both in tissue biopsy/cytology and liquid biopsy. Although tissue biopsy/cytology was better than liquid biopsy, it is more difficult to perform and more invasive.
Furthermore EGFR mutant patients undergoing rebiopsy can present different resistance mechanisms, which reflects intratumoral and intertumoral heterogeneity, as well as dynamic changes in the relative populations of resistant clones over time.8
Despite the retrospective single center nature and small sample of our study, it is the first to present rebiopsy data of EGFR mutated NSCLC patients in Portugal and it is in line with other similar studies.9,10 Eun Kyong Goag et al. also reported a sample with a similar prevalence of EGFR mutations, with 561% in exon 19 del 34,1% in L858R or L861Q (compared with 47,8% of del19 and 34,8% in L858R in our sample) and the T790M mutation was identified in 43.9% patients with exon 19 del as the most significant factor affecting T790M mutation development (hazard ratio: 6.875, P = 0.014). Similarly, our detection rate of T790M was 44% after the first tissue rebiopsy and the T790M mutation was detected in 50% of patients with an initial del19 mutation (compared with 65% in Goag’s study) and in 26% of patients with and an initial L858R mutation (vs. 21,5%).9 Similarly in another Japanese study, the T790M mutation was also more frequent with an exon 19 deletion mutation (63%) than in those with a L858R mutation (38%) (p = 0.035).10 Prospective, multicenter studies are needed to validate these findings.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interestThe authors have no conflicts of interest to declare.