Sedatives have been increasingly used to improve patient comfort during flexible bronchoscopy (FOB). Due to its rapid-onset, anxiolytic and amnestic properties, midazolam is one of the most commonly used sedatives.
ObjectivesTo evaluate the effect of sedation with midazolam, including patient tolerance, complications and its potential use on a daily routine basis.
MethodsA multi-centre, prospective, randomized, placebo-controlled study was made on 100 patients submitted to FOB in two Pulmonology Departments. Midazolam (0.05mg/kg) was administered to patients in Group 1 and saline solution (0.9% NaCl) to patients in Group 2, 5min before the procedure. The Hospital Anxiety and Depression Scale (HADS-A) was used to determine patient anxiety level. Subjective questionnaires concerning main fears and complaints were answered before and after FOB.
ResultsMean age was 56.0±14.1 years; 66% were male. Most (65%) patients had low score (<7) in HADS-A scale with no difference between groups. No significant differences were seen between groups concerning FOB duration, procedures, lidocaine dosage and complications. Systolic blood pressure during and after FOB was significantly higher (p<0.003) in Group 2.
Patients in Group 1 experienced less cough (32% vs 56%; p=0.03) and dyspnoea (2% vs 34%; p<0.001) than in Group 2, while nausea (6% vs 18%; p>0.05) and pain (4% vs 12%; p>0.05) were not statistically different.
Willingness to repeat the exam was reported in all patients in Group 1 and in 82% in Group 2 (p=0.003).
ConclusionSedation with midazolam in FOB improved patient's comfort and decreased complaints, without significant haemodynamic changes. It should be offered to the patient on a routine basis.
Os agentes sedativos têm vindo a ser cada vez mais utilizados na broncofibroscopia (BF) para melhorar o conforto do doente. Devido à sua rápida ação, propriedades ansiolíticas e amnésicas, o midazolam é um dos sedativos mais frequentemente usados.
ObjetivosAvaliar o efeito da sedação com midazolam na BF, incluindo a tolerância do doente, complicações e a sua potencial aplicação na prática clínica diária.
Material e MétodosEstudo multicêntrico, prospetivo, randomizado, controlado com placebo, com inclusão de 100 indivíduos submetidos a BF em 2 Serviços de Pneumologia. Doentes do Grupo 1 receberam midazolam (0,05mg/kg) e doentes do Grupo 2 receberam placebo (0,9% NaCl), 5 minutos antes do procedimento. A escala de ansiedade «The Hospital Anxiety and Depression Scale» (HADS-A) foi aplicada para determinar o nível de ansiedade basal do doente. Questionários subjetivos acerca dos principais receios e queixas relativamente à BF foram aplicados antes e depois do exame.
ResultadosMédia de idades de 56,0±14,1 anos; 66% do sexo masculino. A maioria (65%) dos doentes apresentava baixa pontuação (<7) na escala HADS-A, sem diferença entre grupos.
Não se observaram diferenças significativas entre os 2 grupos no que diz respeito à duração da BF, procedimentos realizados, dose total de lidocaína usada e complicações observadas. A pressão arterial sistólica foi significativamente mais elevada (p<0,003), durante e após a BF, nos indivíduos do Grupo 2.
Os doentes do Grupo 1 apresentaram menos tosse (32 vs 56%; p=0,03) e dispneia (2% vs 34%; p<0,001) comparativamente com o Grupo 2, não se registando diferenças significativas relativamente à náusea (6 vs 18%; p>0,05) e à dor (4 vs 12%; p>0,05).
Foi demonstrada recetibilidade em repetir o exame por todos os doentes do Grupo 1 e em 82% dos doentes do Grupo 2 (p=0,003).
ConclusãoA sedação com midazolam na BF aumentou o conforto e diminuiu queixas dos doentes, não se verificando alterações hemodinâmicas significativas. Deve ser oferecida, de forma regular, ao doente submetido a BF.
Sedation in flexible bronchoscopy (FOB) is widely used by pulmonary physicians in Europe and in the USA.1,2 However, to our knowledge, in Portugal its use is not a daily routine practice.
FOB is usually stressful and patients often present fear and anxiety. Discomfort during and after FOB, with persistent cough, pain and shortness of breath, is also common. Several authors have shown that use of sedation ensures greater comfort for the patient and strengthens the willingness to repeat the exam, if necessary. Moreover, it may shorten the duration of the examination, with fewer interruptions, providing more satisfactory conditions for complex techniques (e.g., bronchial brushing and biopsy, trans-bronchial needle aspiration), and therefore better diagnostic results.2–4
The choice of a drug with sedative properties and its dose varies according to the patient's age, associated morbidities, medication and bronchoscopist's personal preference.5 The most commonly used drugs are midazolam and propofol. The former has optimal properties for ambulatory invasive procedures – with rapid onset action, short half-life and few side effects. Some studies have shown that midazolam is well tolerated and associated to greater patient satisfaction, compared to other sedatives.6,7 Others have not reported significant differences, in terms of efficiency and tolerance, between midazolam and opioids, as a single sedative drug during FOB.7 The risk for cardio-respiratory depression should always be considered, although the usually low dosage (<5mg), the continuous cardiac and respiratory monitoring and the existence of an effective antagonist (flumazenil), make it a rare and well-manageable side effect.5,8
Sedation during FOB is, therefore, a common practice worldwide. The potential cardiorespiratory depressor effect, especially when used in double or triple combination, warrants adequate safety measures including continuous monitoring and immediate availability of antagonist drugs.
ObjectiveThe objective of our study was the evaluation of sedation in FOB with midazolam as a daily routine practice in a Bronchology Department. Patient comfort and satisfaction were the primary outcomes. Secondary objectives included hemodynamic changes, side effects and diagnostic yield.
Materials and methodsStudy designA multi-centre, randomized, double-blind, prospective, placebo-controlled study, lasting 3 months was performed from April to June 2009, in two Pulmonology Departments – Centro Hospitalar S. João EPE and Hospital de Braga. It was approved by both Hospital Ethical Committees and all patients were informed and signed consent.
SubjectsWe included 100 patients who underwent FOB during that period. They were randomly assigned to two groups (50 patients each), Group 1 (received intravenous midazolam at a dose of 0.05mg/kg) and Group 2 (placebo group; received intravenous saline solution).
Exclusion criteria were defined as age >80 and <18, respiratory failure (pO2<60 mmHg and/or pCO2<45 mmHg) or renal failure, hemodynamic instability, neurologic disease, platelet count <50,000/mm3, severe COPD (FEV1<50%; respiratory depression and hypoventilation with sedation may cause acute respiratory failure in patients with severe airflow limitation), depression of consciousness, cognitive impairment preventing answering questionnaires.
Study protocolClinical assessment including co-morbidities, usual medication and smoking history was registered. Peripheral venous access and supplementary oxygen were also routine practice. Continuous monitoring of oxygen saturation, heart rate and blood pressure was done.
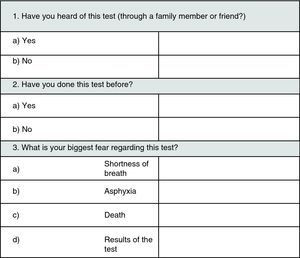
Before administration of midazolam or saline solution, patients answered the Hospital Anxiety and Depression Scale – Anxiety subscale, to evaluate anxiety baseline status before FOB, defined by a numeric scale (from 0 to 27), dividing patients into 3 groups: (1) without psychopathology, (2) borderline and (3) with psychopathology. They were also asked to answer an initial questionnaire about their expectations towards the examination and previous experiences (Fig. 1 – Questionnaire no. 1).
Local anaesthesia of rhinopharyngeal and oropharyngeal region was applied with 10% lidocaine after the administration of midazolam or placebo. Sedation level was determined using the Ramsey Sedation Scale. This scale has the following score: 1 – Anxious or restless; 2 – Cooperative, oriented and tranquil; 3 – Responding to commands; 4 – Brisk response to stimulus; 5 – Sluggish response to stimulus; 6 – No response to stimulus.
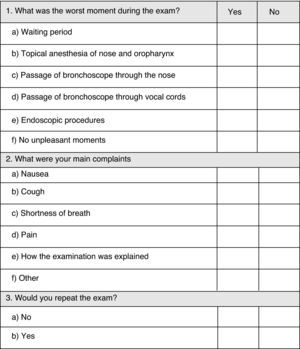
During FOB the following data were registered: lidocaine 2% dose usage; duration of the exam; medication used; endoscopic techniques performed; blood pressure, heart rate and pulse oximetry; complications. One hour after conclusion of FOB, the patient was asked to answer a second questionnaire regarding main complaints and willingness to repeat FOB if necessary (Fig. 2 – Questionnaire no. 2).
Endoscopic procedures were performed by physicians with different levels of experience, including interns under supervision, according to local organization.
Statistical analysisThe SPSS v.17 (Chicago, Illinois, EUA) was used for statistical analysis. The Kolmogorov–Smirnov test was used to assess the normal distribution of variables. The t-student or the Mann–Whitney tests were used for quantitative variables, and Chi-Square and Fisher tests were applied for qualitative variables. Significant statistical difference was considered when p value<0.05.
ResultsOne hundred individuals were randomized, 50 in each group (Group 1 received midazolam and Group 2 received placebo). Mean age was 56±14 years (range 18–79 years); 66% were males. Differences between groups concerning age, sex, smoking history, level of education and FOB indication were not significant (Table 1).
Characteristics of patients in both groups.
| Variable | Total (n=100) | Group 1 (n=50) | Group 2 (n=50) | p value |
| Age, years (mean) | 56.0±14.1 | 55.0±14.4 | 57.1±13.8 | NS |
| Sex (F/M), n (%) | 34 (34)/66 (66) | 18 (36)/32 (64) | 16 (32)/34 (68) | NS |
| Smoking habits, n (%) | ||||
| Smoker | 27 (27) | 14 (28) | 13 (26) | |
| Ex-smoker | 25 (25) | 11 (22) | 14 (28) | NS |
| Non-smoker | 48 (48) | 25 (50) | 23 (46) | |
| Education level, n (%) | ||||
| <4 years | 21 (21) | 12 (24) | 9 (18) | |
| 4–9 years | 58 (58) | 29 (58) | 29 (58) | NS |
| 9–12 years | 10 (10) | 5 (10) | 5 (10) | |
| >12 years | 11 (11) | 4 (8) | 7 (14) | |
| FOB indication, n (%) | ||||
| Malignancy | 29 (29) | 12 (24) | 17 (34) | |
| Infection | 14 (14) | 5 (10) | 9 (18) | NS |
| Interstitial lung disease | 16 (16) | 9 (18) | 7 (14) | |
| Haemoptysis | 16 (16) | 8 (16) | 8 (16) | |
| Other | 25 (25) | 16 (32) | 9 (18) | |
| HADS-A score, n (%) | ||||
| No psychopathology (0–7) | 65 (65) | 34 (68) | 31 (62) | |
| Borderline (8–10) | 20 (20) | 10 (20) | 10 (20) | NS |
| With psychopathology (11–27) | 15 (15) | 6 (12) | 9 (18) | |
| Previous FOB (yes/no), n (%) | 24 (24) | 8 (16) | 16 (32) | NS |
| Midazolam dose, mg (mean) | NA | 2.56±1.61 | NA | NA |
| FOB duration, min (mean) | 13.8±7.0 | 13.6±6.9 | 14.0±7.2 | NS |
| Lidocaine dose, mg (mean) | 308±87 | 302±91 | 313±83 | NS |
| Complications, n (%) | 4 (4) | 1 (3) | 3 (6) | NS |
Most (65%) individuals did not present pathological anxiety on the HADS-A scale (score >7); 20% scored borderline and 15% fulfilled criteria of psychopathology.
Questionnaire 1 disclosed that 51% of patients were not familiar with FOB while for 24% it was not their first experience (no differences between groups). FOB results (41%) and shortness of breath (23%) were the main fears about FOB, while 30% denied any particular concern.
Ramsey Sedation Scale was applied to evaluate sedation level after administration of midazolam or placebo (Table 2). Mean scores were 2.77±1.19 for Group 1 and 1.72±0.50 for Group 2.
Ramsey Sedation Scale.
| Ramsey Sedation Scale | Group 1 (n=50) | Group 2 (n=50) |
| 1 – Anxious or restless | 3 | 15 |
| 2 – Cooperative, oriented and tranquil | 25 | 34 |
| 3 – Responding to commands | 6 | 1 |
| 4 – Brisk response to stimulus | 8 | 0 |
| 5 – Sluggish response to stimulus | 6 | 0 |
| 6 – No response to stimulus | 0 | 0 |
| Incomplete data | 2 | 0 |
Patients in Group 1 received a mean dose of 2.56±1.61mg of midazolam (1.0–5.75mg). Concerning FOB duration and lidocaíne median dosage, no significant differences were observed between groups. Group 1 and Group 2 received a median dosage of lidocaíne of 302±91mg and 313±83mg, respectively. FOB duration was similar in both groups (13.6±6.9 and 14.0±7.2min).
Complications were noted in one patient in Group 1 (oxygen desaturation) and in 3 patients in Group 2 (oxygen desaturation, hypotension and bronchospasm). The administration of flumazenil for midazolam neutralization was not needed at any time.
Hemodynamic and cardiovascular parameters were analyzed before, during and after FOB, and compared between groups (Table 3). At baseline, blood pressure (134/78 vs 138/80mmHg), heart rate (80 vs 82beats/min) and sO2 (98.6 vs 98.6%) were similar for both groups. In Group 1 the mean systolic blood pressure was maintained during and after FOB (135mmHg) whereas in Group 2 it increased from 138mmHg at baseline to 151mmHg during FOB (p=0.001) and 152mmHg at the end (p=0.003). Heart rate increased to a mean level of 91beats/min in Group 1 and 94beats/min in Group 2 during FOB, and 91beats/min in Group 1 and 88beats/min in Group 2, after FOB (p>0.05). No significant changes occurred in mean sO2 level when comparing both groups during and after FOB (Group 1 – 97.0%; Group 2 – 97.6%; p>0.05).
Hemodynamic parameters.
| Variable | Before FOB | During FOB | After FOB | ||||||
| Group 1 | Group 2 | p | Group 1 | Group 2 | p | Group 1 | Group 2 | p | |
| Systolic blood pressure (mmHg) | 134 | 138 | NS | 135 | 151 | 0.001 | 135 | 152 | 0.003 |
| Diastolic blood pressure (mmHg) | 78 | 80 | NS | 77 | 81 | NS | 79 | 84 | NS |
| Heart rate (bpm) | 80 | 82 | NS | 91 | 94 | NS | 91 | 88 | NS |
| Oxygen saturation (%) | 98.6 | 98.6 | NS | 97.2 | 98.2 | NS | 97.0 | 97.6 | NS |
Questionnaire no. 2 evaluated patient's main complaints and willingness to repeat the exam if needed (Table 4). Eighteen patients did not have any complaints at all and 15 of those belonged to Group 1 (p=0.002). Topical nose anaesthesia was defined as the worst moment of the procedure by 50 patients (20 of Group 1 and 30 of Group 2; p>0.05). Passage of the bronchoscope through nose (p>0.05) and vocal cords (p=0.017) were frequently identified as the main complaint and at a higher rate in the placebo group. Complaints about endoscopic procedures, nausea and pain were not significantly different between groups. Dyspnoea (Group 1 – 2%; Group 2 – 34%; p<0.001) and cough (Group 1 – 32%; Group 2 – 56%; p=0.003) were defined as the worst complaint by a significantly higher number of patients in Group 2 than in Group 1. Fifteen patients complained about the waiting period and seven about the way the exam was explained, with no difference between groups. The willingness of patients to repeat the procedure was 100% for Group 1 and 82% for Group 2, with statistical difference (p=0.003).
Complaints post-FOB (Questionnaire no. 2).
| Variable | Group 1 (n=50) | Group 2 (n=50) | p value |
| Question no. 1: Worst moment | |||
| Waiting period, n (%) | 10 (20) | 5 (10) | NS |
| How the procedure was explained, n (%) | 3 (6) | 4 (8) | NS |
| Topical anesthesia of nose/oropharynx, n (%) | 20 (40) | 30 (60) | NS |
| Passage of bronchoscope through nose, n (%) | 5 (10) | 12 (24) | NS |
| Passage of bronchoscope through vocal cords, n (%) | 4 (8) | 13 (26) | 0.017 |
| Endoscopic techniques, n (%) | 3 (6) | 9 (18) | NS |
| No unpleasant moments, n (%) | 15 (30) | 3 (6) | 0.002 |
| Question no. 2: Main complaint | |||
| Nausea, n (%) | 3 (6) | 9 (18) | NS |
| Cough, n (%) | 16 (32) | 28 (56) | 0.030 |
| Dyspnoea, n (%) | 1 (2) | 17 (34) | <0.001 |
| Pain | 2 (4) | 6 (12) | NS |
| Question no. 3: Willingness to repeat FOB | 50 (100) | 41 (82) | 0.003 |
Midazolam is widely used in FOB for mild sedation, due to its pharmacological properties.9 In our study we found that administration of midazolam at a low dosage (0.05mg/kg) to patients undergoing FOB significantly improved their comfort compared to a placebo-control group. It also decreases fear and rejection in repeating the procedure. These findings are consistent with other clinical trials, with similar design, using randomized groups, that compared midazolam with placebo.6,10
In a randomized study with 69 patients, comparing midazolam vs alfentanil, no significant differences were observed between groups in relation to patient comfort and tolerance, ease in performing FOB and oximetry. Alfentanil was associated to less cough and midazolam to patient preference.11 Another study observed that the combined regimen of midazolam with alfentanil was associated to increased risk of desaturation during FOB when compared to either drug alone,12 with no significant advantage to patient comfort.
Studies comparing midazolam with propofol also showed no differences concerning comfort and hemodynamic changes, although the propofol group recovered conscience faster.13,14
Recently newer sedative drugs have been used, namely fospropofol which has a lower peak concentration compared to propofol.5 However more studies are necessary and it is not yet available in Portugal.
Patients randomized in midazolam and control group had similar demographic characteristics. Baseline anxiety or other possible psychiatric disorder was evaluated by the HADS-A scale. The objective was to determine whether results might be influenced by baseline psychological status and to ensure that there was no significant difference between the two groups. In fact, most patients did not present psychopathology according to HADS-A scale, with a similar distribution in both groups.
Surprisingly we found a significant percentage of patients who claimed no particular fear about FOB (30%) when answering questionnaire no. 1. In fact, fear before the test is almost universal.10 This may be influenced by various factors such as level of information made available15 or the kind of approach (written vs verbal).
Parameters evaluated before FOB and those related to the procedure itself such as the duration, endoscopic techniques, lidocaine usage, and complications were not influenced by mild sedation with midazolam. Conscious and light sedation was accomplished with a low dose of midazolam (mean 2.6mg) with only 6 patients scoring >4 in Ramsey Sedation Scale, as described in other reports.6 None reached score 6 (no response to stimuli) and flumazenil was not used at any time. This absence of sedation-related complications should contribute to its generalized use in FOB.
Hemodynamic changes during FOB often result in increasing blood pressure and heart rate and a decrease in sO2. The procedure itself or the use of sedative agents may account for desaturation episodes.1,8 In our study we observed a stabilizing effect of the systolic blood pressure during and after FOB in patients receiving midazolam (135 vs 152mmHg; p<0.003). These data are consistent with other findings16 where hypertension is widely reported in the non-sedated group. No significant differences were seen regarding heart rate and sO2 between groups. Two episodes of oxygen desaturation occurred, one in each group.
Patient comfort during the procedure was assessed through a written questionnaire one hour after the end of FOB. Pain (Group 1 – 4%; Group 2 – 12%) and nausea (Group 1 – 6%; Group 2 – 18%) were low in both groups with no statistical difference (p>0.05). However, patients who received midazolam experienced much less dyspnoea (2% vs 34%; p<0.001) and cough (32% vs 56%; p=0.003) and there was also a significant percentage of patients in this group who denied unpleasant events (30% vs 6%; p=0.002). Patient tolerance to FOB was greater when midazolam was given and, in these patients, willingness to repeat the procedure was universal (p=0.003), which is a predictor of patient satisfaction.
Concluding, we conducted a study that, in our opinion, offers high-level evidence for the routine use of midazolam in FOB, because it included a considerable number of patients based on two Pulmonology Departments of different Portuguese hospitals. This placebo-controlled trial showed that patients receiving midazolam have better tolerance and are more likely to repeat the procedure. This mild sedation is associated with hemodynamic stability and absence of severe complications which should also encourage its routine use in patients with no contraindications. Updates regarding other drugs that may be used to ensure patient comfort have been published17 and, in fact, the existing literature supports the safety and effectiveness of this approach when the proper agents are used in appropriately selected patients.18,19
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Rui Rolo, Sedação com midazolam na broncofibroscopia – estudo prospectivo, Rev Port Pneumol 2012. http://dx.doi.org/10.1016/j.rppneu.2012.03.002.