Most of the studies of the pathophysiology of Obstructive Sleep Apnea-Hypopnea Syndrome (OSAHS) focus on the collapsibility and obstruction of the upper airways. The aim of our study was the investigation of small airways’ function in patients with OSAHS.
Materials and methodsWe studied 23 patients (mean age, 51.6 years) diagnosed with mild to severe OSAHS, without comorbidities and 8 controls (mean age, 45.9 years). All subjects underwent full polysomnography sleep study; spirometry and maximum flow/volume curves while breathing room air and a mixture of 80%He-20%O2. The volume of equal flows (VisoV⋅) of the two curves and the difference of flows at 50% of FVC (ΔV˙max50) were calculated, as indicates of small airways’ function.
ResultsThe results showed that VisoV⋅ was significantly increased in patients with OSAHS compared with controls (18.79±9.39 vs. 4.72±4.68, p=0.004). No statistically significantly difference was found in ΔV˙max50% (p=0.551); or the maximum Expiratory flow at 25–75% of FVC (p=0.067) and the maximum expiratory flow at 50% of FVC (p=0.174) breathing air.
ConclusionsWe conclude that at the time of the diagnosis of OSAHS, the function of the small airways is affected. This could be due to breathing at low lung volumes and the cyclic closure/opening of the small airways and may affect the natural history of OSAHS. The findings could lead to new therapeutic implications, targeting directly the small airways.
Sleep is a complex, active and in many ways a different condition from alertness. During sleep, the control of breathing is subject to an altered regulation. In addition, the mechanics of breathing change, the resistance of the upper airways increases and the functional residual capacity decreases. This reduction in lung volumes leads to an increased tendency towards upper airway obstruction and contributes to the reduction of flow during inhalation, although the exact mechanisms of these findings have not been described in detail.1,2
The most common sleep disorder-OSAHS-has been studied and reviewed extensively.3,4 Among the important physiological traits causing OSAHS are: (1) pharyngeal anatomy/collapsibility, (2) ventilatory control system gain (loop gain), (3) the ability of the upper airway to dilate/stiffen in response to an increase in ventilator drive, and (4) arousal threshold.4 Although, large and small airways are part of the same anatomical compartment of the lungs, the studies of the pathophysiology of OSAHS have focused primarily on the function of the upper airways.
The aim of our study is to investigate the function of small airways in patients with OSAHS, and to compare it with healthy controls.
To the best of our knowledge, this is the first investigation of the small airways in OSAHS showing that their function is affected.
Materials and methodsSubjects23 subjects (13 males, 10 females; mean age, 51.6 years) with a diagnosis of mild to severe OSAHS, without comorbidities and 8 controls (3 males, 5 females; mean age, 45.9 years) (Table 1) were included in the study. Patients and controls were recruited at the Sleep Disorders Center of the Medical School of University of Crete from June 2013 to July 2014, complaining of sleep-related symptoms.
Characteristics of patients and controls subjects.
| Patients (n=23) | Controls (n=8) | p-Value | |
|---|---|---|---|
| Sex | 0.433 | ||
| Male | 13 (56.5) | 3 (37.5) | |
| Female | 10 (43.5) | 5 (62.5) | |
| Age | 51.6±12.7 | 45.9±15.5 | 0.310 |
| Smoking | 0.676 | ||
| Ex-smokers | 9 (39.1) | 2 (25.0) | |
| Nonsmokers | 14 (60.9) | 6 (75.0) | |
| ESS | 10.8±5.5 | 5.6±3.4 | 0.020 |
| BMI (kg/m2) | 31.6±5.7 | 24.6±3.4 | 0.003 |
| Neck circumference(cm) | 40.0±3.4 | 36.5±5.0 | 0.036 |
| Mild OSAHS (5≤AHI<15) | 5 (21.7) | ||
| Moderate OSHAS (15≤AHI<30) | 10 (43.5) | ||
| Severe OSHAS (AHI≥30) | 8 (34.8) | ||
| FEV1(L/min) | 93.2±13.7 | 104.8±11.3 | 0.070 |
| FVC (L/min) | 97.9±15.6 | 106.0±14.1 | 0.262 |
| FEV1/FVC | 79.7±5.2 | 84.2±3.9 | 0.062 |
AHI: Apnea-Hypopnea Index, ESS: Epworth Sleepiness Scale, BMI: Body Mass Index.
Subjects having any of the following were excluded from the study:
(1) Severe obesity (Body Mass Index (BMI) >40kg/m2), (2) Current smokers or ex-smokers >30pys, (3) Patients with concomitant pulmonary disease (e.g. Chronic Obstructive Pulmonary Disease (COPD), asthma, interstitial lung disease, active pulmonary tuberculosis, (4) Patients previously diagnosed with sleep-related disorder, (5) Patients who have clinically significant renal, cardiovascular, neurological, endocrine, immunological, psychiatric, gastrointestinal, hepatic, or hematological abnormalities, (6) Pregnancy.
Protocol- 1.
Physical examination was performed and medical history was taken, including sleep disorders symptoms. The daytime somnolence was evaluated with the Epworth scale and measurements of: Weight (kg) and Height (m) were performed. The BMI (kg/m2) was calculated, and the neck circumference (cm) was measured at the height of the cricothyroid cartilage.
- 2.
Patients and control subjects underwent polysomnographic sleep study where the following parameters were recorded: Electroencephalography, Electrooculogram, Electromyography in submental muscle and anterior tibia muscles bilaterally, Electrocardiogram, microphone should be placed over the trachea or on the side of the neck (from AAST), the detection of air flow with oral-nasal thermistor and pressure transducer, oxyhemoglobin saturation by finger pulse oximeter, the movement of thoracic and abdominal wall with special elastic belts of thorax and abdomen for the recording of the respiratory effort and body position.
The diagnosis of OSAHS was established when the Apnea-Hypopnea Index (AHI) ≥5 and its severity was distinguished as:
- •
Mild: 5≤AHI<15
- •
Moderate: 15≤AHI<30
- •
Severe: AHI≥30
- •
- 3.
Patients and controls underwent spirometry, according to the ERS/ATS guidelines, in sitting and supine position.
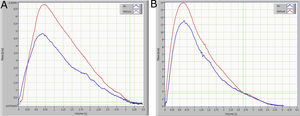
The small airways’ function was assessed using the Helium-Oxygen flow–volume method. The subject was seated and instructed to perform maximal expiratory flow-volume curves while breathing room air or a mixture of 80% He and 20% O2. Flow-volume curves while breathing He-O2 were measured after the subject had breathed in at least three Vital Capacity (VC) inspirations of the He-O2 mixture (heliox). The two curves obtained from breathing room air and the He-O2 mixture, were superimposed visually from Total Lung Capacity (TLC). A difference of less than 5% in FVC between breathing room and the He-O2 mixture was considered acceptable (the mean of our data was <3%). When the V˙−V curves did not have identical FVC, they were superimposed from Residual Volume (RV)5,6 (Fig. 1). The volume at which the flow became identical was defined as VisoV⋅. The VisoV⋅ was expressed as a percentage of the Forced Vital Capacity (FVC), according to the method of Dosman and colleagues6 (Fig. 1). The value of ΔV˙max50 was calculated from the equation ΔV˙max50=(V⋅Emax50He−V⋅Emax50air/V⋅Emax50air)×100, and expressed as a percentage of V˙Emax50 while breathing room air.6
Statistical analysisDescriptive analysis of the participants’ characteristics was conducted. The normality assumption of the continuous variables was assessed via the One Sample Kolmogorov – Smirnov Test and through graphical inspection. Continuous variables are presented as mean±standard deviation (SD) or as median and 25th–75th percentiles. Differences between continuous variables were assessed via the independent samples T-test and the independent Mann–Whitney U Test where appropriate. Differences between categorical variables were assessed via the Fisher's exact test. Linear regression analysis was used on the association of OSAHS with V˙isoV, adjusted for BMI. Wilcoxon matched-pairs signed-ranked test was used for comparison between sitting and supine posture. For all tests, a p-value<0.05 was considered statistically significant. All statistical tests were performed using the SPSS 22.0 (IBM Corporation, Illinois, US) and Stata 13.0 (StataCorp, College Station, TX, US).
ResultsDemographic data, smoking habits, pulmonary function tests (PFTs), and sleep related parameters of the patients and control subjects, are shown in Table 1.
Statistically significant differences between patient and controls, were found regarding BMI, Epworth Sleepiness Scale (ESS) and neck circumference (p-value<0.05).
The parameters of small airways’ function are shown on Table 2.
Parameters of small airways’ function in patients and control subjects in sitting position.
| Patients (n=23) | Controls (n=8) | p-Value | |
|---|---|---|---|
| MMEF25–75 | 77.5±25.5 | 100.8±16.3 | 0.067 |
| MEF50 | 86.3±28.1 | 103.8±21.0 | 0.174 |
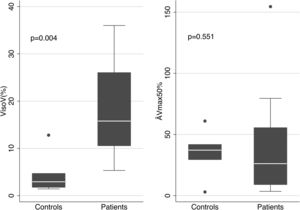
| VisoV˙(%) | 18.8±9.4 | 4.7±4.7 | 0.004 |
| aΔV˙max50% | 26.2 (9.1, 55.6) | 37.2 (29.5, 41.8) | 0.551 |
The mean values of the basic parameters of small airways’ function (VisoV˙% and DV˙max50% in patients and controls are shown in Fig. 2. VisoV˙ was statistically significantly increased in patients with OSAHS compared to controls (18.79±9.39 vs. 4.72±4.68, p=0.004). OSAHS was significantly associated with higher VisoV˙ [beta (95% CI)=12.118 (1.51, 22.73)], independently of BMI. No statistically significantly relationship was found between VisoV˙ and ESS or neck circumference in OSAHS patients.
ΔV˙max50% was not statistically significantly different {26.2 (9.1, 55.6) vs. 37.2 (29.5, 41.8), p=0.551}, although a trend to decrease was seen in patients.
Non-significant differences in the mean values of FVC, Forced Expiratory Volume in 1s (FEV1), FEV1/FVC, Maximal (mid-) Expiratory Flow at 25–75% of FVC (MMEF25–75) and Maximal (mid-) Expiratory Flow at 50% of FVC (MMEF50) were found between patients with OSAHS and the controls in sitting position (Tables 1 and 2).
In patients with OSAHS, the mean values of pulmonary function tests in supine position had significantly decreased statistically from their values in sitting position (Table 3).
Pulmonary function tests in sitting and supine position, in patients.
| Sitting position | Supine position | p-Value | |
|---|---|---|---|
| FEV1 (L/min) | 92.5 (87.0, 101.0) | 85.5 (74.0, 98.0) | 0.0021 |
| FVC (L/min) | 95.0 (91.0, 109.0) | 91.0 (79.0, 99.0) | 0.004 |
| FEV1/FVC | 80.0 (78.5, 81.7) | 78.6 (71.7, 83.3) | 0.004 |
| MMEF25-75 | 74.0 (58.0, 98.7) | 63.5 (50.0, 82.0) | 0.0039 |
| MEF50 | 86.0 (64.0, 108.0) | 86.5 (58.0, 91.0) | 0.0157 |
Wilcoxon matched-pairs signed-ranked test.
Small airways are the peripheral membranous bronchioles, 2mm less in diameter. They make up a very large area which is difficult to approach functionally, clinically and therapeutically.7,8
The main finding of the present study is that small airways’ function is affected in OSAHS, as the VisoV˙ increases, despite normal baseline spirometric values. These findings, to the best of our knowledge, are reported for the first time.
The pathogenesis of OSAHS is multifactorial; anatomical and functional changes of the upper airway and its musculature result in partial or complete and recurrent obstructions of the upper airway during sleep. Anatomical changes contribute to the reduction of the diameter of the upper airway while the functional changes to the increased collapsibility and susceptibility to obstruction and these changes may interact, i.e., the structural changes affect the functional ones and vice versa.9
It is well known, that during sleep the pattern of breathing is variable and that sleep stages significantly affect this pattern.10 In addition, in OSAHS it is reported that lung volumes may continuously vary during NREM sleep and this may contribute to passive collapse of the upper airways.11 However, during sleep, the common finding is the reduction of FRC or the End Expiratory Lung Volume (EELV), even if it fluctuates. Breathing at low lung volumes results in reduction of the elastic restoring force of the lungs (elastic recoil) and reduces radial traction exerted by the lung parenchyma on the airways to keep them open with reduced flow resistances.12 This occurs even in normal sleeping subjects and may be enhanced abnormally in sleeping disorders.1
Additionally, in obese subjects with OSAHS, it has been shown that reduced pulmonary volumes, secondarily lead to narrowing and obstruction of the upper airways.13 Jordan et al showed that when genioglossus muscle fails to stabilize breathing in OSAHS, this may be related to reduced lung volume.14
Therefore, the majority of the pathophysiological studies have focused on the collapsibility of the upper airways.
In contrast, our study found an affected function at the site of the small airways. We speculate that this could be due to opening and closure of the small airways caused by the abrupt changes in the airway pressure at low lung volumes. It is well known, that during the obstruction of the upper airways the airway pressure increases in order to reopen the collapsed site and to initiate inspiratory flow. When this has been achieved, a significant and abrupt drop in the airway pressure is seen. These changes in airway pressure are transmitted into the whole respiratory system and may cause collapsibility and flapping at the site of the membranous small airways. In support of this argument there are studies in animals showing that ventilation at low lung volumes leads to an abnormal successive opening and closure of small airways.15 Such periodic continuous occlusion and opening, creates conditions for inflammation and oxidative stress in the regions of the final bronchioles and alveolar sacks, which means destruction of lung parenchyma.15
In 2002, D’Angelo E, Milic-Emili J et al, reported that small-volume ventilation causes peripheral airway injury and increases airway resistance in normal rabbits and this is probably due to cyclic opening and closing of the peripheral airways.16
In addition, Yalcin HC et al, in an in vitro model of airway reopening showed significant epithelial cell injury.17 Moreover, Zerah-Lancner et al, found a significant decrease in the FEV1/FVC ratio, in the V50 and V25, as the severity of OSAHS increases, in 170 patients undergoing a sleep study.18
Baydur et al, showed expiratory flow limitation in COPD and OSAHS patients, but their results could not distinguish the two cohorts.19
Finally, in agreement with our results are those of Abdeyrim et al and Cai et al who used the impulse oscillation technique to show that the peripheral airway resistance increased in obese OSAS patients.20,21 Moreover, Abdeyrim et al showed that Reactance at 5Hz correlated with peripheral airway resistance and with the decreased FRC.20
In addition, Avraam et al reported that reductions in FVC while supine and with increased body weight may contribute to worsening of OSAHS.22
In accordance with these studies, our results show a significant decrease in patient pulmonary function values in supine position from the sitting, (Table 3) which strengthens our argument that breathing at low lung volumes may affect negatively small airways function.
Moreover, Heinzer et al showed that the increase in pulmonary volumes reduces sleep disturbances and improves sleep architecture in patients with OSAHS during non-REM sleep.23
Review and critique of the methodIn 1974, Hutheon et al introduced the volume of isoflow as a new test for the detection of small-airway dysfunction.24 During the terminal 10% to 15% of VC, the flow rates during helium-oxygen and air breathing are identical; this is the volume of isoflow point.25 The isoflow phenomenon is seen because flow in the small airways is laminar and so independent of gas density.26VisoV˙ is expressed as a percent of VC and occurs in normal nonsmoking subjects at 10% to 15% of VC from RV.26 The reduction in radius is making the phenomenon of laminar flow to accurate at higher lung volumes (increase in VisoV˙) and this is considered as a malfunction of small airways.27
Despas et al reported that early manifestation of peripheral airway obstruction can be detected in patients with mild asthma using the He-O2 mixture.5 Moreover, Dosman et al showed that the use of Heliox during a maximal expiratory flow-volume maneuver was capable of detecting functional abnormalities in smokers at a stage when spirometric indices were within the normal range.6
This technique has been used by Siafakas et al to investigate small airways’ function in acromegaly.28 It has been also used by our department, for the investigation of small airways’ function in non-respiratory diseases such as inflammatory bowel diseases, with interesting results.29 In agreement with the present study they found alterations only in the VisoV˙ index and not in the ΔV˙max50 and considered that ΔV˙max50 could not be a sensitive index for the early detection of small airway dysfunction.30
It is well known that, the small airways of the lung, the so-called “quiet zone”,30,31 is a difficult anatomical area to approach and therefore for studying its function. Multiple methods have been proposed to study this area of the lung, including complicated and invasive techniques. Nevertheless, there is a lack of a globally acceptable methodology to investigate small airways.32,33
There are some studies that criticize density-dependence tests for their variability and their validity for detecting narrowing of the small airways,34 however the majority agree that those tests correlate well with the small airways function.35,36
Although, BMI was statistically different between the two groups, regression analysis showed that VisoV˙ was statistically higher in OSAHS patients, independently of BMI. Similarly, Abdeyrim et al reported that FRC and Expiratory Reserve Volume reductions were independent from BMI in OSAHS patients.20
Limitation of our study is the quite small sample size. Only 23 patients with OSAHS were studied and 8 controls. However, the results of VisoV˙ clearly separated patients from controls (Fig. 2).
Finally, preliminary results have shown a trend towards improvement in small airways parameters in OSAHS patients after therapy with CPAP. Thus, it would be worth investigating the effects of treatment of OSAHS on the function of small airways in the future.
ConclusionsThe main finding of the present study is that patients with OSAHS showed a dysfunction of the small airways, since the VisoV˙ index was increased, despite their normal baseline spirometric values.
This is probably due to the reduction of the lung volumes, the cycle opening and closure of the small airways and subsequent inflammation and oxidant stress.
The function of the small airways may affect the natural history of OSAHS and these findings could lead to new therapeutic implications. However, larger studies are needed to verify our results.
Compliance with ethical standardsThis study was supported by an unrestricted grant to University of Crete by Elpen Hellas, with no other involvement in the study.
Ethical approvalAll procedures performed in the study were in accordance with the ethical standards of the Research Ethics and Deontology Committee of the University of Crete, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
All subjects have signed an Informed Consent Form prior to any investigation.
Authors contributionsKG: She was involved in all parts of this work, the design of the study, the experiments, the analysis of the data, and the interpretation of the results, the drafting and writing of the manuscript.
SS: Contributed in the design of the study, in the analysis of the data and supervised the polysomnographic sleep studies.
GV: Contributed in the acquisition and the analysis of the data and in their statistical interpretation.
VL: He was involved in the design of the study, in the analysis of the data and supervised the Heliox experiments.
NT: contributed in the design of the study, organized the Heliox experiments and was involved in the analysis of the data.
NS: He is the senior investigator of the study involved in all aspects: design, analysis, interpretation of the results and supervised the writing of the manuscript.
All authors have read and approved the final manuscript.
Conflicts of interestThe authors declare that they have no conflict of interest.
The authors have no relationship with the tobacco industry or its affiliates and subsidiaries that benefited any of the manuscript authors or the tobacco industry in its promotion of tobacco products.
Preliminary results of this study have been previously reported, as an abstract, at the ERS International Congress 2017 Eur Resp J 2017 50: PA2309.