The present study investigated the relationship between central hemodynamics and lung function and the response to an acute bout of exercise in COPD.
MethodsBased on the severity of COPD, moderate group (MOD, n = 12) and more mild group (MLD, n = 12) underwent central hemodynamic assessments pre- and post-peak exercise.
ResultsIn the entire cohort (n = 24), central diastolic blood pressure (cDBP) was associated with pulmonary function. Post-exercise, cDBP remained elevated (p < 0.01), however, peripheral diastolic blood pressure (pDBP) was reduced (p = 0.02). Prior to exercise, the MOD showed higher cDBP and heart rate (HR) than the MLD (p = 0.02 and p = 0.01, respectively), but no difference in central aortic/arterial stiffness (p > 0.05). These findings remained similar post-exercise.
ConclusionCentral diastolic blood pressure is linked to pulmonary function in COPD and it is elevated after exercise-induced reductions in pDBP. Central diastolic blood pressure is higher in the MOD than the MLD, however, there was no difference in central aortic/arterial stiffness between groups.
There is a growing prevalence of chronic obstructive pulmonary disease (COPD) globally and 15.2% of Americans suffer from this disease.1 The lungs and cardiovascular systems are intimately linked and it is well known that smoke inhalation is a major risk factor for vascular disease. In addition, the lungs and heart compete for intrathoracic space and alterations in lung mechanics associated with lung disease – hyperinflation – and shifts in intrathoracic pressure due to lung disease likely also impact central hemodynamics.2–4 Furthermore, pulmonary vascular remodeling occurs in COPD, which consequently influences pulmonary vascular pressures.5,6 Therefore, the present study investigated the relationship between lung function and central aortic hemodynamics in COPD, and the response of central aortic hemodynamics to acute exercise.
MethodsThe present study was reviewed and approved by Mayo Clinic Institutional Review Board. Twenty-five patients with COPD provided written informed consent and underwent pulmonary function testing (PFT), quality of life questionnaire assessment, as well as quantification of blood pressure (BP) and heart rate (HR). Subsequently, subjects were divided into either a more moderate disease group (MOD) or a mild group (MLD) based on scoring of COPD severity using lung function. Severity score was determined by quantifying lung diffusing capacity for carbon dioxide (DLCO), forced vital capacity (FVC), forced expiratory flow between 25 and 75% of FVC (FEF25−75), forced expiatory volume-one second (FEV1), maximum voluntary ventilation (MVV), and then ranking subjects from 1st (the most severe) to 24th (the least severe) based on percent predicted value for each parameter. The rankings for each of the 5 parameters were then averaged for each subject, and severity status determined by the average rank such that the 1st to 12th ranked subjects were defined as severe (MOD, n = 12) and the 13th to 24th ranked subjects were defined as moderate (MLD, n = 12). Table 1 demonstrates the subjects’ characteristics.
Subjects characteristics.
| Parameters | The MOD (n = 12) | The MLD (n = 12) | t-test |
|---|---|---|---|
| Demographic | |||
| Age (years) | 66.5 ± 8.9 | 68.9 ± 9.3 | p = 0.66 |
| Sex (M/F) | 8 / 4 | 5 / 7 | |
| Height (cm) | 170.5 ± 10.5 | 166.7 ± 12.9 | p = 0.43 |
| Weight (kg) | 95.3 ± 21.8 | 82.8 ± 17.5 | p = 0.14 |
| BMI (kg/m2) | 32.6 ± 5.7 | 29.8 ± 5.7 | p = 0.25 |
| Current smoking status (Y/N) | 3 / 9 | 0 / 12 | |
| Cardiovascular | |||
| Resting HR (bpm) | 81.3 ± 9.5a | 70.6 ± 8.8a | p = 0.02 |
| pSBP (mmHg) | 122.7 ± 20.0 | 122.3 ± 10.7 | p = 0.96 |
| pDBP (mmHg) | 75.8 ± 10.6 | 69.0 ± 7.2 | p = 0.08 |
| cSBP (mmHg) | 132.5 ± 17.9 | 127.5 ± 20.6 | p = 0.53 |
| cDBP (mmHg) | 87.6 ± 12.8a | 75.0 ± 8.8a | p = 0.01 |
| Pulmonary | |||
| FVC (%predicted) | 70.5 ± 20.4 | 80.1 ± 10.2 | p = 0.16 |
| FEV1 (%predicted) | 49.8 ± 18.4a | 65.0 ± 7.2a | p = 0.01 |
| FEV1/FVC | 56.0 ± 10.4a | 64.6 ± 8.3a | p = 0.04 |
| FEF25−75 (%predicted) | 22.0 ± 14.9a | 37.3 ± 13.0a | p = 0.01 |
| MVV (%predicted) | 41.0 ± 19.5 | 54.3 ± 12.0 | p = 0.06 |
| Medications | |||
| Inhaled Beta agonist (n) | 12 | 12 | |
| Inhaled Anticholinergic (n) | 9 | 6 | |
| Inhaled steroid (n) | 9 | 7 | |
| Oral steroid (n) | 2 | 2 | |
| Questionnaire | |||
| COPD-GOLD (class I/II/II/IV) | 0/3/8/4 | 1/11/0/0 | |
| Quality of life | 45.8 ± 25.5 | 40.7 ± 19.6 | p = 0.39 |
Body mass index (BMI), peripheral systolic blood pressure (pSBP), peripheral diastolic blood pressure (pDBP), forced vital capacity (FVC), forced expiratory volume-one second (FEV1), forced expiratory flow between 25% and 75% of FVC (FEF25−75), Maximum voluntary ventilation (MVV) and GOLD disease classification for COPD.
Peripheral systolic blood pressure (pSBP) and diastolic blood pressure (pDBP) was measured manually, while central systolic pressure (cSBP), central diastolic pressure (cDBP), central pulse pressure (cPP), pressure of the incident wave (P1), augmentation pressure (AP), pulse wave velocity (PWV), augmentation index (AIx) were assessed via central arterial pressure waveform analysis (Sphygmocor, AtCor Medical, Australia). All subjects underwent a symptom-limited incremental exercise test on a cycle ergometer and the wattage was increased by 10−15 watts every minute to exertional exhaustion. During exercise, respiratory gas exchange, heart rate (HR), pSBP, pDBP, oxygen saturation (SaO2), rate of perceived exertion (RPE) and dyspnea were assessed. After exercise, subjects were at rest for 20 min before a repeat central hemodynamic assessment was obtained.
To examine the relationships between central aortic hemodynamics and pulmonary function measures in all subjects (n = 24), correlation coefficient analysis was conducted. To compare group differences (MOD vs MLD) at pre-exercise, independent t-test and the Mann-Whitney U non-parametric t-test were conducted. To examine an exercise effect, a repeated-measures analysis of variance (ANOVA) was conducted to observe alterations in central hemodynamic parameters from pre- to post-exercise.
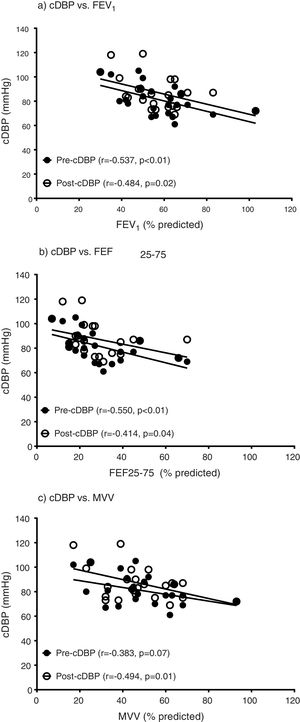
ResultsEntire groupFrom pre-exercise to post-exercise, COPD (n = 24) demonstrated significant increases in cSBP (pre vs. post, 126.9 ± 19.8 vs. 133.6 ± 15.8, p = 0.01) and cDBP (pre vs. post, 74.8 ± 8.4 vs. 81.5 ± 9.5, p < 0.01) with a significant decrease in pDBP (pre vs. post, 68.2 ± 7.6 vs. 64.9 ± 8.4, p = 0.02). However, other central hemodynamic parameters including AP, PWV and AIx were not altered (p > 0.05). In addition, pulmonary function parameters including FEV1, FEF25−75 and MVV were associated with cDBP at pre- and post-exercise (Fig. 1), however, these measures of lung function were not related to cSBP, AP, PWV and AIx (p > 0.05).
The relationships between central diastolic blood pressure and parameters of pulmonary function. Central diastolic blood pressure (cDBP), forced expiratory volume-one second (FEV1), forced expiratory flow between 25% and 75% of FVC (FEF25–75), maximum voluntary ventilation (MVV). a) The relationships between pre-exercise cDBP and pre-exercise FEV1 (r = −0.499, p = 0.01) and post-exercise cDBP and pre-exercise FEV1 (r = −0.484, p = 0.02). b) The relationships between pre-exercise cDBP and pre-exercise FEF25–75 (r = −0.530, p = 0.01) and post-exercise cDBP and pre-exercise FEF25–75 (r = −0.414, p = 0.04). c) The relationships between pre-exercise cDBP and pre-exercise MVV (r = −0.349, p = 0.09) and post-exercise cDBP and pre-exercise MVV (r = −0.493, p = 0.01).
At pre-exercise, the MOD showed higher resting HR and cDBP than the MLD (p = 0.02 and p = 0.01, respectively, Table 1). In addition, the MOD showed a trend of higher pDBP (p = 0.08, Table 1). However, there was no significant difference between groups in cSBP, cPP, P1, AP, PWV and AIx (p > 0.05). During exercise, the MOD had lower VO2peak (ml/kg/min, p = 0.04) and lower respiratory exchange ratio (RER, p = 0.01) than MLD. In addition, there was a trend that the MOD demonstrated a lower ratio of mixed expired CO2 and end tidal CO2 (PECO2/PETCO2) than the MLD, an index of ventilation distribution (p = 0.09).
Post-exercise, COPD (n = 24) showed increases in cSBP and cDBP and a decrease in pDBP, however, this phenomenon was not significantly different between the MOD and the MLD (p > 0.05). In addition, other central hemodynamic parameters including P1, AP, PWV and AIx were not significantly changed or different between groups (p > 0.05).
DiscussionEntire groupIn the present study, more impaired pulmonary function was related to a higher cDBP in patients with mild to moderate COPD. This relationship was not altered post exercise. After exercise, cDBP in both patient groups remained higher than pre-exercise, however, pDBP was lower than pre-exercise. These data may suggest that obstructive lung disease is associated with higher cDBP and more hemodynamic challenges arose centrally rather than peripherally after exercise.
MOD vs MLD groupsThe MOD had poorer pulmonary function, reduced exercise capacity and a trend of impaired gas exchange (PECO2/PETCO2) relative to the MLD. Additionally, the MOD had a higher cDBP, HR and pDBP than the MLD, but there was no significant difference in AP, PWV and AIx between groups. It is not clear mechanistically the cause of the isolated elevation in cDBP without changes in AP, PWV and AIx. We speculate that central/arterial stiffness might be similar between groups, however, given the higher HR and likely greater degree of obstruction and hyperinflation in the more severe group, i.e., greater mechanical constraint, the coupling of cardiac hemodynamics and cDBP maybe be differentially influenced. However, exercise did not impact differently on alterations in cSBP, cDBP and pDBP between groups.
ConclusionsCentral diastolic blood pressure is elevated in COPD patients, linked to disease severity, and remains elevated after exercise-induced reductions in peripheral diastolic blood pressure. However, these observations occurred similarly between mild and more moderate disease.
Conflicts of interestThe authors have no conflicts of interest to declare.