The 2009 pandemic influenza A (H1N1) (i.e., Pandemic Influenza) is an acute, infectious illness caused by the influenza A (H1N1) 2009 virus. This disease involves respiratory, gastrointestinal and systemic symptoms along with a high incidence occurring at a paediatric age.
ObjectiveTo study the epidemiology, approach and complications of Pandemic Influenza in the paediatric population of a third-level hospital in Lisbon, Portugal between September and December 2009.
Materials and methodsA retrospective study of children who had received the influenza A (H1N1) 2009 virus test by real time reverse transcriptase-polymerase chain reaction (RT-PCR) was included. The following parameters were analysed: number of tests, days of illness, sex, outcome, age, symptoms, hospitalisation and reason for testing. The distribution and test results were compared with the Pandemic Influenza activity in Portugal. Moreover, among the confirmed cases of infection, the need for hospitalisation, risk factors, severity, chest radiography, treatment and complications were also examined.
ResultsA total of 351 tests were performed, on average, 2.6 days after initial symptoms, which included 71.8% outpatients and 30% children younger than 3 years of age. Overall, 54.4% of the tests were positive for the influenza A (H1N1) 2009 virus and the main comorbidities were respiratory and cardiovascular in nature. One hundred ninety-one cases were confirmed by laboratory studies, and 13.6% required hospitalisation, which lasted an average of 2.7 days. In 82.2% of the cases, the severity was mild, with fever and cough as the most frequent symptoms at 91.9% and 93.7%, respectively. Therapy with the antiviral drug, oseltamivir, was implemented in 35.6% of the cases. Additionally, oseltamivir was used in 12 infants younger than 1 year in age, including a 1-month-old infant with no observed side effects.
DiscussionThe epidemiological data obtained are consistent with the published national and international studies. The scientific information available and the recommendations of the Directorate-General for Health contributed to the uniformity of the approaches and the successful outcome.
A pandemia por vírus influenza A (H1N1) 2009 (i.e., Pandemia de Gripe A) é uma doença infeciosa aguda causada pelo vírus influenza A (H1N1) 2009. Esta patologia apresenta sintomas respiratórios, gastrointestinais e sistémicos, tendo elevada incidência na idade pediátrica.
ObjetivoEstudar a epidemiologia, a abordagem e as complicações da Pandemia de Gripe A na população pediátrica de um hospital de nível iii em Lisboa, Portugal, entre setembro e dezembro de 2009.
Material e métodosEstudo retrospetivo dos processos das crianças que realizaram pesquisa do vírus influenza A (H1N1) 2009 por reverse transcriptase-polymerase chain reaction. Analisaram-se os seguintes parâmetros: número de testes, dia de doença, sexo, resultado, distribuição etária, sintomatologia, internamento e motivo para realização do teste. A distribuição e os resultados dos testes foram comparados com a atividade gripal da Pandemia de Gripe A em Portugal. Nos casos de infeção confirmada, estudaram-se também a necessidade de internamento, fatores de risco, gravidade, radiografia de tórax, tratamento e complicações.
ResultadosRealizaram-se 351 testes, em média 2,6 dias após o início dos sintomas, dos quais 71,8% em ambulatório e 30% em crianças com idade inferior a 3 anos. No total, 54,4% dos testes foram positivos para o vírus influenza A (H1N1) 2009 e as principais comorbilidades foram de natureza respiratória e cardiovascular. Cento e noventa e um casos foram confirmados laboratorialmente e 13,6% necessitaram de internamento, com duração média de 2,7 dias. Em 82,2% dos casos, a gravidade foi ligeira, constituindo a febre e a tosse a sintomatologia mais frequente, presente em 91,9% e 93,7% respetivamente. A terapêutica com o antiviral, oseltamivir, foi implementada em 35,6% dos casos. O oseltamivir foi utilizado em 12 crianças com idade inferior a um ano, incluindo uma com um mês de idade, sem registo de efeitos secundários.
DiscussãoOs dados epidemiológicos obtidos estão conformes com os estudos nacionais e internacionais publicados. A informação científica disponibilizada e as recomendações de Direção-Geral de Saúde contribuíram para a uniformização de condutas e evolução clínica favorável.
The 2009 pandemic influenza A (H1N1) (i.e., Pandemic Influenza) is an acute, infectious illness caused by the influenza A (H1N1) 2009 virus, involving respiratory, gastrointestinal and systemic symptoms.1 Compared with the seasonal influenza, Pandemic Influenza had an increased infection rate among children and adolescents. In this age group, most cases consisted of mild to moderate forms of the disease, although severe and fatal cases are also described.1,2
Infection with the influenza A (H1N1) 2009 virus causes a wide spectrum of clinical syndromes from feverless upper respiratory infection to fulminate viral pneumonia.1 The typical flu-like symptoms, including fever, cough, sore throat, headache, rhinorrhoea and myalgia, are predominant. Additionally, gastrointestinal symptoms (e.g., nausea, vomiting and diarrhoea) are also frequently observed.1,3–8
The following conditions represent risk factors for complications and serious manifestations: under 5 years of age, pregnancy, cardiovascular disease, chronic lung, renal and liver disease, immunosuppression, severe obesity (i.e., BMI>25 up to 10 years in age, BMI>35 from 10 to 18 years in age), haemoglobinopathy, neuromuscular disease and metabolic disorder.1,3,9–11
Laboratory diagnosis can be performed by real time reverse transcriptase-polymerase chain reaction (RT-PCR) from samples of nasal and/or oropharyngeal secretions. This test shows high sensitivity and can be accomplished in time for a clinical decision.1,3–6,11
Treatment with oseltamivir, when initiated early and cautiously, could improve the prognosis by reducing the need and duration of hospitalisation as well as the risk of progression to severe and fatal forms of the disease.1,3,12,13
In Portugal, the Pandemic Influenza response was organised in accordance with the National Pandemic Plan from the Directorate-General for Health (Direcção-Geral de Saúde [DGS]). During the 2009 pandemic influenza A (H1N1) virus infection, the DGS has estimated an attack rate of 10–15%, with a peak incidence at weeks 47 and 48 of 2009. Of the total number of reported cases (n=192,294), 0.6% required hospitalisation, and 0.1% were admitted to a hospital intensive care unit (ICU). During the mitigation phase, a phase that began on the 21 of August 2009 and focused on clinical diagnosis, vigilance and/or treatment of patients,10 30% of admissions occurred in children under the age of ten. The crude mortality rate was 1.17/100,000 inhabitants, and of the 124 deaths, six occurred in individuals under the age of 15.10
Limited data are available on disease characteristics, treatment with oseltamivir and outcome of children with Pandemic Influenza, namely in Portugal. Therefore, it is important to collect and critically analyse clinical information that may be useful in evaluating the impact and management of Pandemic Influenza.
ObjectivesThe purpose of this study is to analyse the epidemiology, approach and complications of Pandemic Influenza in the paediatric population of a third-level hospital in Lisbon, Portugal between September and December 2009, which represents the period of increased Pandemic Influenza activity.
Materials and methodsA retrospective study was conducted on the cases of children who had received the influenza A (H1N1) 2009 virus test by RT-PCR and attended the emergency service of a paediatric service at a third-level hospital in Lisbon, Portugal. A third-level hospital is characterised as a public hospital that has highly differentiated skills, human and technical resources with national or inter-regional responsibilities. In this study, only one test was performed on each child. The following parameters were assessed: number of tests, days of illness, sex, outcome, age distribution, symptoms, hospitalisation and the basis on which detection was performed. The distribution and the test results were compared with the Pandemic Influenza activity in Portugal. In laboratory-confirmed cases of infection, the following parameters were also analysed: treatment, hospitalisation, risk factors, severity (according to the criteria established by the official recommendations from DGS),14 chest radiograph and complications. Data were collected from computerised medical records from the Department of Paediatrics and the official forms from DGS used to request the test.15 Data analysis was performed using Microsoft Office Excel 2007® and the chi-square and Mann–Whitney tests. A p value of less than 0.05 was considered statistically significant.
ResultsThe search for the influenza A (H1N1) 2009 virus by RT-PCR in the paediatric population of a third-level hospital in Lisbon, Portugal
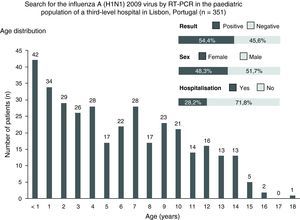
During the study period, 351 tests were performed, on average, 2.6 days (range, 1–10 days) after the appearance of the initial symptoms. Of these patients, 51.7% (n=181) were male, and 54.4% (n=191) tested positive for the virus. The median age was 5 years old (range, 1 month to 18 years), and 30% (n=105) of the individuals were younger than 3 years in age (Fig. 1).
The most frequent symptoms were fever (96.6%), cough (87.7%), rhinorrhoea (60.4%), sore throat (40.5%), headache (38.7%), vomiting (31.9%), myalgia (27.6%) and diarrhoea (18.8%).
Of the total number of tests given, 71.8% (n=252) were performed on an outpatient basis (Fig. 1). Of these, 65.5% (n=165) showed a positive result. Additionally, most children exhibited flu-like syndromes (92%). A total of 99 admitted children were tested, and 26.3% (n=26) of these tests were positive. These tests were required particularly for patients with pneumonia (29%) and bronchiolitis (25%). The difference in the number of positive tests between the outpatient (65.5%) and under hospitalisation (26.3%) groups was statistically significant (p<0.01).
The main indications for performing the study were the following: prevalent chronic lung disease, including asthma and recurrent wheezing in daily therapy with inhaled corticosteroids (n=58); cardiovascular disease (n=23); and immunosuppression (n=10). The major indications related to the contacts (i.e., an individual known to have been sufficiently near an infected person to have been exposed to the transfer of infectious material) were pregnancy (n=44) and children less than 12 months of age (n=30). In some cases, there was more than one reason for the test.
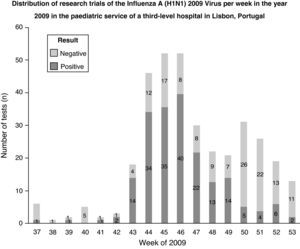
Regarding the Pandemic Influenza activity in Portugal in 2009, it was found that during the peak of influenza activity, an increased number of tests were ordered, and the majority of the results were positive. When the Pandemic Influenza activity decreased, the majority of tests were negative (Fig. 2).
Laboratory-confirmed cases of the influenza A (H1N1) 2009 virus infection in the paediatric population of a third level hospital in Lisbon, Portugal
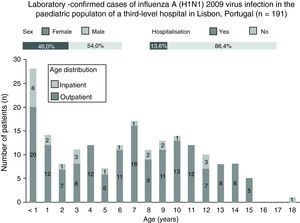
During the study, 191 cases of the influenza A (H1N1) virus infection were confirmed on a laboratory basis, of which 54.0% (n=103) were male, 13.6% (n=26) required hospitalisation and 22% were under 2 years of age (Fig. 3). Of the total amount of confirmed cases, the median age was 7 years (range, 1 month to 18 years). In the outpatient setting, the median age was 7 years (range, 6 months to 15 years), and for hospitalised patients, the median age was 3 years of age (range, 1 month to 18 years); this difference was statistically significant (p<0.05).
In children with Pandemic Influenza, the most frequent symptoms were fever (97.9%), cough (93.7%), rhinorrhoea (57.6%), headache (55.0%), sore throat (49.2%) and myalgia (37.7%). Additionally, cough, sore throat, headache and myalgia occurred more often in confirmed cases (p<0.01). Fever, rhinorrhoea and gastrointestinal symptoms (i.e., vomiting (32.5%), diarrhoea (15.2%) and nausea (9.9%)) were also more frequent, but the difference between positive and negative cases was not statistically significant.
Under 2 years of age (n=50), asthma and recurrent wheezing (n=25) and cardiovascular disease (n=15) were the most common risk factors among the confirmed cases.
The severity of the disease was mild in 82.2% (n=157) of the cases, and the remaining cases presented moderate forms. Additionally, there were no serious cases reported. Among the outpatient cases, mild forms predominated (89.0%), and the inpatient cases were predominately of the moderately severe form (61.5%).
Of the 191 laboratory-confirmed cases, oseltamivir therapy was administered to 35.6% (n=68) with side effects, including vomiting in 5.9% (n=4). This antiviral drug was established in 28.6% (n=47) of children treated as outpatients and in 80.8% (n=21) of those under hospitalisation; this difference was statistically significant (p<0.01). All hospitalised children that were medicated with oseltamivir (n=21) began therapy within the first 24h of hospitalisation, and 71.4% (n=15) began the treatment within the first 48h of the disease. Twelve infants younger than 1 year of age (17.6%), including a 1-month-old infant, were treated with oseltamivir with no record of any side effects.
Of the 26 children admitted, 65.4% (n=17) underwent chest radiography at admission. Of these, 42.3% (n=7) had a radiologic pattern of bilateral interstitial pneumonia. The average length of hospitalisation was 2.7 days (range, 1–12 days), and one child had a mild pericardial effusion, which was treated without sequelae.
DiscussionThe RT-PCR test for the influenza A (H1N1) 2009 virus was useful in clinical practice, and in most cases, yielded results in time for clinical decision.16 The test was performed on average 2.6 days after initial symptoms, reflecting public awareness of the Pandemic Influenza and prompted the use of health services at an early stage. This allowed for sampling early during the course of the disease, which is ideal to identify the virus and improve the therapeutic approach.17,18 The authors consider it necessary to perform studies to evaluate the demand on available healthcare resources and the cost-effectiveness of health strategies during the Pandemic Influenza.
Regarding the Pandemic Influenza activity in Portugal in 2009, during the peak of influenza activity, an increased number of tests were ordered, and the majority of test results were positive. This result can reflect the predominance of the influenza A (H1N1) 2009 virus during this period. When the Pandemic Influenza activity decreased, the majority of test results were negative. Other studies are needed to understand whether other infectious agents, such as syncytial respiratory virus, increased when Pandemic Influenza activity decreased.
Two main reasons could explain the difference between a positive test on an outpatient (65.5%) and hospitalisation (26.3%). First, it was necessary to exclude Pandemic Influenza in hospitalised children with respiratory tract infection, not only to take infection control measures but also to determine the possible need for therapeutic intervention in positive cases.3,4 Second, atypical forms of presentation are described in children, e.g., without fever, which were not neglected in hospitalised patients.3,5
Reflecting the guidelines of the DGS as well as the risk factors described, our study showed that recurrent wheezing, asthma, cardiovascular disease, immunosuppression, pregnancy of a close contact and children under 5 years of age were the main reasons for testing. Additionally, almost one-third of the tests were performed in children under the age of 3 years.1,3,9–11
According to findings in the literature, the majority of Pandemic Influenza cases consist of mild-to-moderate and self-limited forms, which is understandable because most did not require hospitalisation (86.4%).19
Among outpatients, the median age was 7 years old, and the mild forms prevailed. In contrast, hospitalised children had a median age of 3 years, and the moderate forms predominated. The difference in severity and age of children treated in outpatient clinics and in the hospital was statistically significant. Under 5 years of age and the clinical notion of severity were the main determinants of hospitalisation, which is consistent with that described in other series.4,9,10,20,21
Flu-like symptoms, including fever, cough, rhinorrhoea, headache and sore throat, were the most frequent in the clinic. Given the paediatric age group involved, it is understandable that myalgias and arthralgias did not constitute as common signs as in adults, possibly due to the difficulty children have with describing these symptoms to caretakers. The percentage of children with Pandemic Influenza who developed gastrointestinal symptoms was not statistically significant; however, the percentage of vomiting (32.5%), diarrhoea (15.2%) and nausea (9.9%) are consistent with the data described in other publications.1,19
Oseltamivir therapy was performed in 35.6% of confirmed cases, which was comparable to that by DGS for cases in Portugal (32%).10 This antiviral drug was safe, and vomiting occurred in only 5.9% of subjects, and in no cases was it necessary to discontinue therapy. Oseltamivir was used in 12 infants younger than 1 year in age, including a 1-month-old infant, with no observed side effects. We consider that the prevention recommendations of some adverse events, such as taking medication with food, may have contributed to the rate of adverse effects.3 The safety and effectiveness of antiviral therapy in terms of disease progression should be further confirmed in randomised controlled studies.
The percentage of children receiving oseltamivir was higher (80.8%) among hospitalised patients than among outpatients (28.6%); p<0.01. This reflects the predominance of moderate severity forms observed among hospitalised children as well as the fact that the not prescribing oseltamivir or prescribing oseltamivir after 48–72h could compromise prognoses.13 The administration of oseltamivir within the first 48h of disease onset in 71.4% of hospitalised children may have contributed to the improved prognoses. The advantages described for this drug, particularly in terms of reducing the length of hospitalisation and progression to the severe forms of the disease, may have contributed to the average length of stay (2.7 days), which is lower than the average hospital stay in Portugal (DGS – 4.6 days) and in other studies.1,3,9,19,20
The five hospitalised patients that did not receive therapy with oseltamivir were admitted because of incoercible vomiting without any other symptoms or severity signs. These children were asymptomatic and discharged 24h after admission.
The abnormalities found on a chest radiography were nonspecific and similar to those found for other viral infections, which does not allow us to infer the infection aetiology.4,20
The clinical outcome was favourable in all cases, even in the event of a slight pericardial effusion that resolved spontaneously and without sequelae in one case. This complication has been described in the literature.3 There were also no serious respiratory complications or need for ICU admission.
The Paediatric Service followed the Directorate-General for Health published guidelines on diagnosis, surveillance and treatment of Pandemic Influenza cases.
There are several limitations to our study. Our study had a small sample corresponding to only one hospital in Lisbon, Portugal; therefore, the results cannot be generalised to other paediatric populations or other geographical areas. The analysis of the laboratory-confirmed cases most certainly underestimated the total number of cases of Pandemic Influenza that had occurred during the study period. We did not evaluate and compare the disease course of children treated either with or without the antiviral drug.
ConclusionIn general, the epidemiological data obtained and the disease approach used were consistent with other national and international published studies. The scientific information available and the recommendations from the DGS contributed to the uniformity of the approaches and successful outcome of cases reported in the population under the study.
Conflicts of interestThe authors have no conflicts of interest to declare.
We would like to acknowledge the staff of the Department of Clinical Pathology and the Department of Paediatrics.
Please cite this article as: Malveiro D, et al. Pandemia por vírus influenza A (H1N1) 2009: experiência de um serviço de pediatria num hospital de nível iii em Lisboa, Portugal. Rev Port Pneumol. 2012. http://dx.doi.org/10.1016/j.rppneu.2012.01.006.